No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark
🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇸🇰 Slovakia🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of America
and more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark
🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇸🇰 Slovakia🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of America
and more
Fervex for adults powder for o...
$24.79 Original price was: $24.79.$22.49Current price is: $22.49.


Lasolvan solution for inhalati...
$27.99 Original price was: $27.99.$25.39Current price is: $25.39.
Strepsils Intensive lollipops with honey and lemon 8.75 mg each 16 pcs
$20.69 Original price was: $20.69.$18.79Current price is: $18.79.
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, Slovakia, South Korea, Switzerland, United Kingdom, United States and more
In stock
Strepsils Intensive Lollipops with Honey and Lemon are an effective and soothing solution for sore throat relief. Each lollipop contains 8.75 mg of flurbiprofen, a powerful anti-inflammatory agent that targets pain and swelling directly at the source. Combined with the comforting flavors of honey and lemon, these lollipops provide both therapeutic relief and a pleasant taste, making them ideal for adults and children alike.
Categories: Cold and flu
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Pharmacological properties
Pharmacodynamics: flurbiprofen is an NSAID that has a powerful analgesic, antipyretic and anti-inflammatory effect by inhibiting the synthesis of prostaglandins.
Alleviation of pain, soreness in the throat area, as well as reduction of swelling in the throat is observed 30 minutes after taking the lollipop, the duration of action is 2-3 hours.
Analgesic and anti-inflammatory activity is due to inhibition of COX enzyme and inhibition of prostaglandin synthesis. The drug has a local effect. Equally suppresses the effect of prostaglandins E 2 and E 2a due to the reduction of endoperoxidase, which catalyzes the transformation of arachidonic acid into cyclic endoperoxides.
Pharmacokinetics. C max of flurbiprofen in the blood plasma is observed 30-40 minutes after the resorption of the lollipop in the oral cavity. Cmax of flurbiprofen after the use of a lollipop is reached faster than after swallowing an equivalent dose, but the concentration levels in both cases are similar. Flurbiprofen is rapidly distributed in the body. The drug is actively metabolized by methylation and hydroxylation with subsequent elimination by the kidneys. The main metabolites of the drug are 4′-oxy-flurbiprofen and 3′-oxy-4’methoxy-flurbiprofen. Approximately 70% of the dose is excreted in the urine after 24 hours. T ½ is 3-6 hours.
Indication
For short-term symptomatic relief of sore throat in adults and children 12 years of age and older.
Application
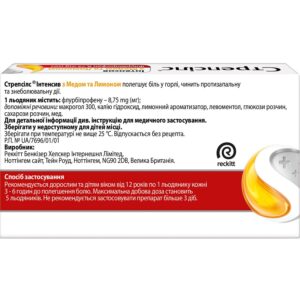
Popsicles are absorbed in the oral cavity until completely dissolved. adults and children over 12 years old take 1 lollipop every 3-6 hours until pain relief. the maximum daily dose is 5 lollipops.
The lowest effective dose is used for the minimum period necessary to relieve symptoms. If the symptoms do not disappear, worsen or last more than 3 days, it is necessary to consult a doctor.
It is not recommended to use the drug for more than 3 days.
During resorption, the lollipop should be moved throughout the oral cavity to prevent irritation of the mucous membrane at the site of resorption.
In elderly patients, due to limited clinical experience, no general dose recommendations can be made at this time. In elderly patients, there is an increased risk of severe side effects.
Contraindication
Hypersensitivity to flurbiprofen or any component of the drug.
Hypersensitivity reactions (eg asthma, bronchospasm, rhinitis, angioedema or urticaria) after the use of acetylsalicylic acid or other non-steroidal anti-inflammatory drugs.
Recurrent gastric ulcer / bleeding in the anamnesis or in the exacerbation phase (two or more clear episodes of exacerbation, confirmed by characteristic clinical manifestations) and intestinal ulcers.
History of gastrointestinal bleeding or perforation, severe colitis, hemorrhagic or hematopoietic disorders associated with previous NSAID treatment.
Severe heart failure, severe renal failure, severe liver failure.
The last trimester of pregnancy.
Side effects
Hypersensitivity reactions to non-steroidal anti-inflammatory drugs have been reported, which manifested as:
- non-specific allergic reaction and anaphylaxis;
- reactivity of the respiratory tract, for example BA, exacerbation of BA, bronchospasm, shortness of breath;
- various skin reactions, such as itching, urticaria, angioneurotic edema, exfoliative and bullous dermatoses (including epidermal necrolysis and erythema multiforme) were rarely noted.
Edema, hypertension, and heart failure have been reported in association with NSAID therapy. Clinical studies and epidemiological data indicate that the use of some NSAIDs (especially in high doses and with long-term treatment) may be associated with a slightly increased risk of arterial thrombotic complications (for example, myocardial infarction or stroke). There are insufficient data to exclude such a risk in the case of lozenges with flurbiprofen 8.75 mg.
Below is a list of side effects observed with short-term use of flurbiprofen in over-the-counter doses.
(Very common: ≥1/10; common: ≥1/100 to 1/10; infrequent: ≥1/1000 to 1/100; rare: ≥1/10,000 to 1/1000; very rare: 1 / 10,000, frequency unknown: it is impossible to estimate the frequency from the available data.)
From the blood and lymphatic system: unknown – anemia, thrombocytopenia.
From the side of the immune system: rarely – anaphylactic reactions.
Psychiatric disorders: infrequently – insomnia.
From the side of the cardiovascular and cerebrovascular system: it is not known – swelling, hypertension and heart failure.
From the side of the nervous system: often – dizziness, headache, paresthesia; infrequently – drowsiness.
From the side of the respiratory system, organs of the chest and mediastinum: often – irritation in the throat; infrequently – exacerbation of asthma and bronchospasm, shortness of breath, wheezing, bubbles in the oropharynx, pharyngeal hypoesthesia.
From the digestive system: often – diarrhea, ulcers in the mouth, nausea, pain in the mouth, paresthesias of the mouth, pain in the oropharynx, discomfort in the mouth (feeling of heat, burning or tingling in the mouth); infrequently – flatulence, abdominal pain, constipation, dry mouth, dyspepsia, flatulence, glossodynia, dysgeusia, oral dysesthesia, vomiting.
From the side of the liver and biliary tract: unknown – hepatitis.
From the side of the skin and subcutaneous tissue: infrequently – various skin rashes, itching; unknown – severe forms of skin reactions, such as reactions of the bullous type, including Stevens-Johnson syndrome and toxic epidermal necrolysis.
General disorders and local reactions: infrequently – pyrexia, pain.
In case of unwanted reactions, you should stop the treatment and consult a doctor.
Special instructions
Manifestations of side effects can be reduced by short-term use of the minimum effective dose necessary to eliminate symptoms.
In elderly patients, the frequency of adverse reactions caused by the use of NSAIDs increases, especially bleeding from the gastrointestinal tract or perforations, which can be fatal.
Bronchospasm can occur in patients with bronchial asthma or allergic diseases now or in the anamnesis.
It is not recommended to use lozenges with flurbiprofen in parallel with other NSAIDs, including selective COX-2 inhibitors.
Systemic lupus erythematosus and systemic connective tissue diseases – increased risk of aseptic meningitis.
Symptoms of kidney failure: nephrotoxicity. NSAIDs have been reported to cause nephrotoxicity in various forms, including interstitial nephritis, nephrotic syndrome, and renal failure, especially when used in combination with multiple analgesic drugs and in case of long-term habitual use. The use of nonsteroidal anti-inflammatory drugs can lead to a dose-dependent decrease in prostaglandin production and provoke renal failure. The greatest risk of this reaction exists in patients with renal failure, heart failure, impaired liver function, patients taking diuretics, and the elderly. Renal function should be monitored in such patients. However, this effect is usually not observed with short-term, limited use of drugs such as flurbiprofen lozenges.
Violation of liver function. Violation of liver function from mild to moderate severity.
Effect on cardiovascular and cerebrovascular system. It is necessary to start using the drug with caution (after consulting a doctor) in patients who have had high blood pressure and/or heart failure, as fluid retention, high blood pressure, and edema have been reported when using NSAIDs. Conducted clinical research and data from epidemiological studies indicate that the use of some NSAIDs (especially in high doses and for a long time) increases the risk of developing arterial thrombotic complications (for example, myocardial infarction or stroke). There are insufficient data to exclude such a risk in the case of using 5 lozenges per day.
Manifestations from the nervous system. Headache caused by painkillers: in case of long-term use of analgesics or non-compliance with recommendations, a headache may occur, which should not be treated with increased doses of the drug.
Violations of reproductive function in women. The use of flurbiprofen can impair fertility in women, so this drug is not recommended for women who are trying to conceive. It is necessary to consider the expediency of canceling this medicinal product for women who have difficulty getting pregnant or are undergoing an infertility examination.
Effect on the gastrointestinal tract. Gastrointestinal bleeding, ulceration, or perforation, which may be fatal, has been reported with all NSAIDs at any stage of treatment, regardless of the presence of warning symptoms or a history of severe gastrointestinal disturbances. The risk increases with increasing doses of NSAIDs in patients with a history of peptic ulcer disease, especially complicated bleeding or perforation, as well as in the elderly. These patients should be started on the lowest available dose. Combination therapy with protective agents (eg, misoprostol or proton pump inhibitors) is recommended in such patients, as well as in patients requiring concomitant low-dose acetylsalicylic acid or other drugs that may increase gastrointestinal risk. Patients should contact their doctor if they experience any unusual gastrointestinal symptoms (especially gastrointestinal bleeding), particularly at the start of treatment. It should be used with caution in patients receiving concomitant therapy with drugs that increase the risk of ulceration or bleeding, in particular oral corticosteroids, anticoagulants such as warfarin, selective serotonin reuptake inhibitors or antiplatelet agents such as acetylsalicylic acid. If gastrointestinal bleeding or ulceration occurs in patients receiving flurbiprofen, treatment should be discontinued. NSAIDs should be used with caution in patients with a history of gastrointestinal disease (ulcerative colitis, Crohn’s disease), as their condition may worsen.
From the side of the skin and subcutaneous tissue. Very rarely, on the background of taking non-steroidal anti-inflammatory drugs, severe forms of skin reactions can occur, which can be fatal, including exfoliative dermatitis, Stevens-Johnson syndrome and toxic epidermal necrolysis. Flurbiprofen lozenges should be discontinued at the first signs of skin rash, pathological changes in the mucous membranes or any other signs of hypersensitivity.
Infections. Since there have been isolated cases of exacerbation of the infectious inflammatory process (for example, the development of necrotizing fasciitis), which were observed in a temporary connection with the use of systemic NSAIDs as a class, the patient is recommended to immediately consult a doctor in case of signs of bacterial infection or worsening of the condition during therapy with lozenges with flurbiprofen. The need for anti-infective therapy with antibiotics should be considered.
Sugar intolerance. Patients with rare hereditary problems of fructose intolerance, glucose/galactose malabsorption or sucrase-isomaltase insufficiency should not use this medicine.
If symptoms worsen or if new symptoms develop, treatment should be reviewed. If irritation occurs in the oral cavity, the treatment should be discontinued.
Use during pregnancy and breastfeeding
Pregnancy. Inhibition of prostaglandin synthesis may adversely affect pregnancy and/or embryo/fetus development. Data from epidemiological studies indicate an increased risk of miscarriage and congenital heart defects and gastroschisis after using a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk of heart defects increased from 1% to 1.5%. The risk is believed to increase with increasing dose and duration of therapy. In animals, the use of an inhibitor of prostaglandin synthesis during the period of organogenesis led to an increase in the number of cases of various malformations, including those of the cardiovascular system. Flurbiprofen should not be taken during the first two trimesters of pregnancy, unless absolutely necessary. If flurbiprofen is used by a woman who is trying to conceive, or during the I and II trimesters of pregnancy, the lowest possible dose should be used for a short period of time. During the III trimester of pregnancy, all inhibitors of prostaglandin synthesis may present the following risks:
- for the fetus: cardiopulmonary toxicity (characterized by premature closure of the ductus arteriosus and pulmonary hypertension); impaired renal function, which may progress to renal failure accompanied by oligohydramnios;
- for the mother at the end of pregnancy and the newborn: an increase in bleeding time, an antiplatelet effect that can develop even at very low doses, inhibition of uterine contractions, which leads to a delay or increase in the duration of labor.
Thus, flurbiprofen is contraindicated in the III trimester of pregnancy.
Breast feeding. In some studies, flurbiprofen has been detected in breast milk at very low concentrations. It is unlikely to have a negative effect on a breastfed baby. However, due to the possible side effects of NSAIDs in breastfed infants, Strepsils Intensive with Honey and Lemon is not recommended for breastfeeding women.
Fertility There is some evidence that drugs that inhibit the synthesis of prostaglandins / COX can lead to the deterioration of female fertility as a result of the effect on the ovulation process. This effect is reversible upon discontinuation of the drug.
Children. It is not recommended to use the drug in patients under 12 years of age.
The ability to influence the speed of reaction when driving a motor vehicle or working with other mechanisms. Studies of the ability to influence the speed of reaction when driving a motor vehicle or other mechanisms were not conducted.
Interactions
The simultaneous use of flurbiprofen should be avoided with:
other NSAIDs, including selective COX-2 inhibitors: the simultaneous use of two or more NSAIDs should be avoided, as this increases the risk of side effects (especially side effects from the gastrointestinal tract, such as ulcers and bleeding), acetylsalicylic acid (in low doses ), if it was not prescribed by a doctor in low doses (not higher than 75 mg/day), as this increases the risk of side effects.
Flurbiprofen should be used with caution in combination with the following drugs:
anticoagulants: NSAIDs can enhance the effect of anticoagulants such as warfarin;
antiplatelet agents: the risk of gastrointestinal ulcers or bleeding increases;
antihypertensive agents (diuretics, ACE inhibitors, and angiotensin II antagonists): NSAIDs can reduce the effect of diuretics and other antihypertensive agents, as well as increase nephrotoxicity caused by COX suppression, especially in patients with impaired renal function (patients should receive sufficient fluid);
alcohol: increases the risk of adverse reactions, especially bleeding in the gastrointestinal tract;
cardiac glycosides: NSAIDs can aggravate heart failure, reduce the rate of glomerular filtration and increase the level of glycosides in the blood plasma. It is recommended to monitor the patient’s condition and, if necessary, adjust the dose;
cyclosporine: increased risk of nephrotoxicity;
corticosteroids: increase the risk of adverse reactions, especially of the gastrointestinal tract;
lithium: possible increase in the level of lithium in the blood plasma, proper control and, if necessary, dose correction;
methotrexate: the use of nonsteroidal anti-inflammatory drugs within 24 hours before or after the use of methotrexate can lead to an increase in the concentration of this drug and an increase in the severity of its toxic effect;
mifepristone: you should not take NSAIDs for 8-12 days after using mifepristone, because NSAIDs can reduce the severity of the action of mifepristone;
oral antidiabetic drugs: the blood glucose level may vary (it is recommended to strengthen the control of the blood glucose level);
phenytoin: an increase in the level of phenytoin in the blood plasma is possible, so proper monitoring and, if necessary, dose correction are recommended;
potassium-sparing diuretics: simultaneous use can lead to hyperkalemia;
probenecid, sulfinpyrazone, medicinal products containing probenecid or sulfinpyrazone: may cause slow-release flurbiprofen;
quinolone antibiotics: Animal studies show that NSAIDs increase the risk of seizures associated with the use of quinolone antibiotics. Patients taking nonsteroidal anti-inflammatory drugs and quinolones have an increased risk of seizures;
selective serotonin reuptake inhibitors: increased risk of gastrointestinal ulceration or bleeding;
tacrolimus: possible increased risk of nephrotoxicity with simultaneous use of NSAIDs with tacrolimus;
zidovudine: increased risk of hematological toxicity with simultaneous use of NSAIDs with zidovudine.
The studies that were conducted today did not reveal the interaction of flurbiprofen with tolbutamide and antacids.
Overdose
Symptoms most patients who have taken potentially toxic doses of NSAIDs may experience nausea, vomiting, pain in the epigastric region, or very rarely – diarrhea. tinnitus, headache, and gastrointestinal bleeding may also occur. with more severe poisoning, toxic damage to the central nervous system can occur, which manifests itself in the form of drowsiness, sometimes – disturbances, as well as disorientation or coma. with severe poisoning, the development of metabolic acidosis is possible, as well as the prolongation of prothrombin time, probably due to the effect on blood coagulation factors circulating in the bloodstream. possible development of OPN and liver damage. patients may have an exacerbation of the course of the disease.
Treatment may be symptomatic and supportive and may include airway patency, monitoring of heart rate and other vital signs until a stable condition is achieved. Oral use of activated charcoal is recommended in the case of a patient’s application within 1 hour after the use of a potentially toxic dose of the drug. In case of frequent or prolonged muscle spasms, intravenous diazepam or lorazepam should be prescribed. Bronchodilators should be used for BA. There is no specific antidote to flurbiprofen.
Storage conditions
At a temperature not higher than 25 °C.
Be the first to review “Strepsils Intensive lollipops with honey and lemon 8.75 mg each 16 pcs” Cancel reply
You may also like









Reviews
There are no reviews yet.