No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
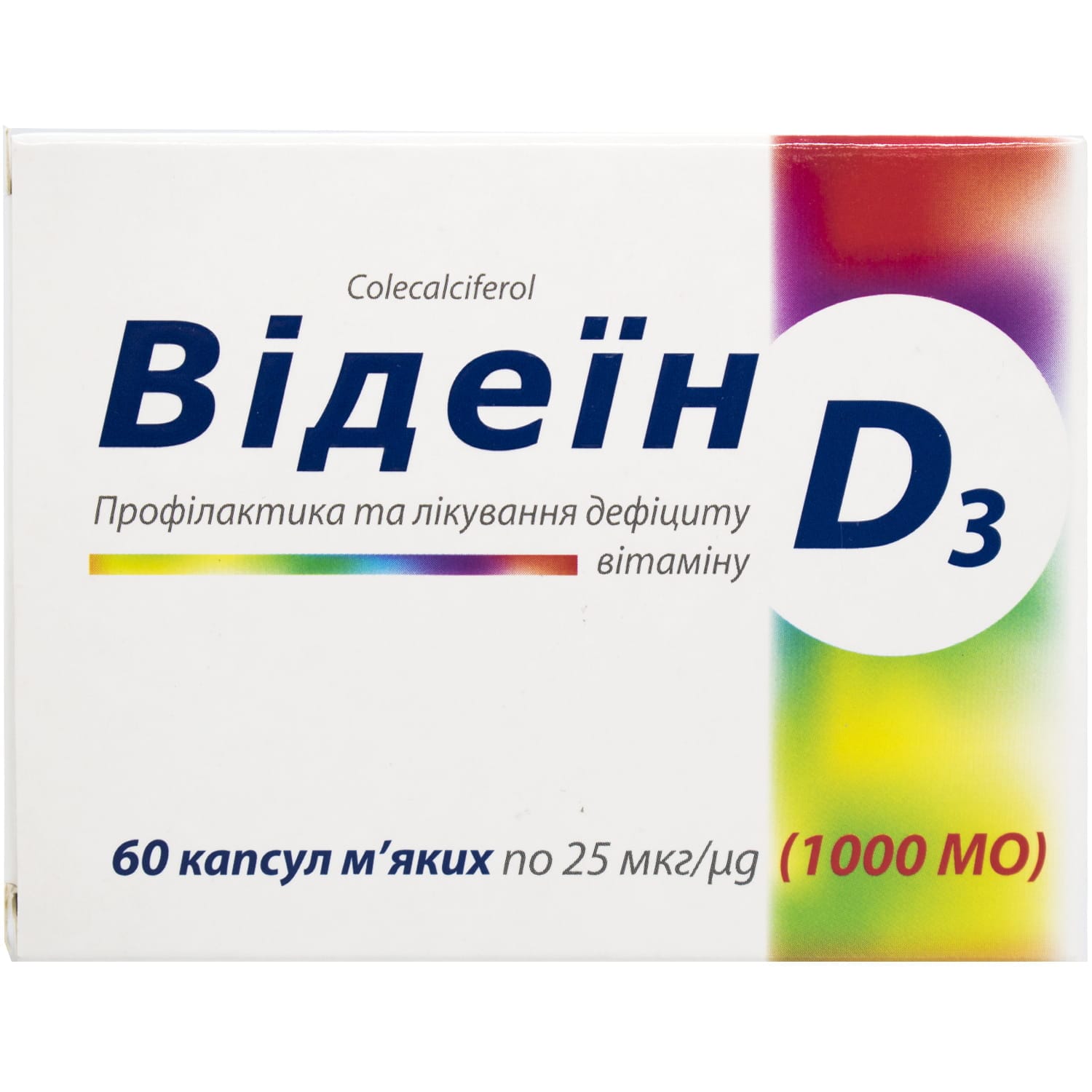
Videin soft capsules 25 mcg (1000 IU), 60 capsules
$19.87
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, South Korea, Switzerland, United Kingdom, United States and more
In stock
Videin soft capsules, 25 µg (1000 IU), for rickets prevention, vitamin D3 deficiency, osteomalacia, and osteoporosis support. 60 capsules.
Categories: Vitamins and supplements
Brand: Kyiv Vitamin Plant
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Soft capsules “Videin” are used for the following indications:
- prevention of rickets, including in premature newborn infants;
- prevention of vitamin D3 deficiency in high-risk patients who do not have malabsorption disorders;
- prevention of vitamin D3 deficiency in malabsorption;
- treatment of rickets and osteomalacia;
- maintenance therapy of osteoporosis.
Composition
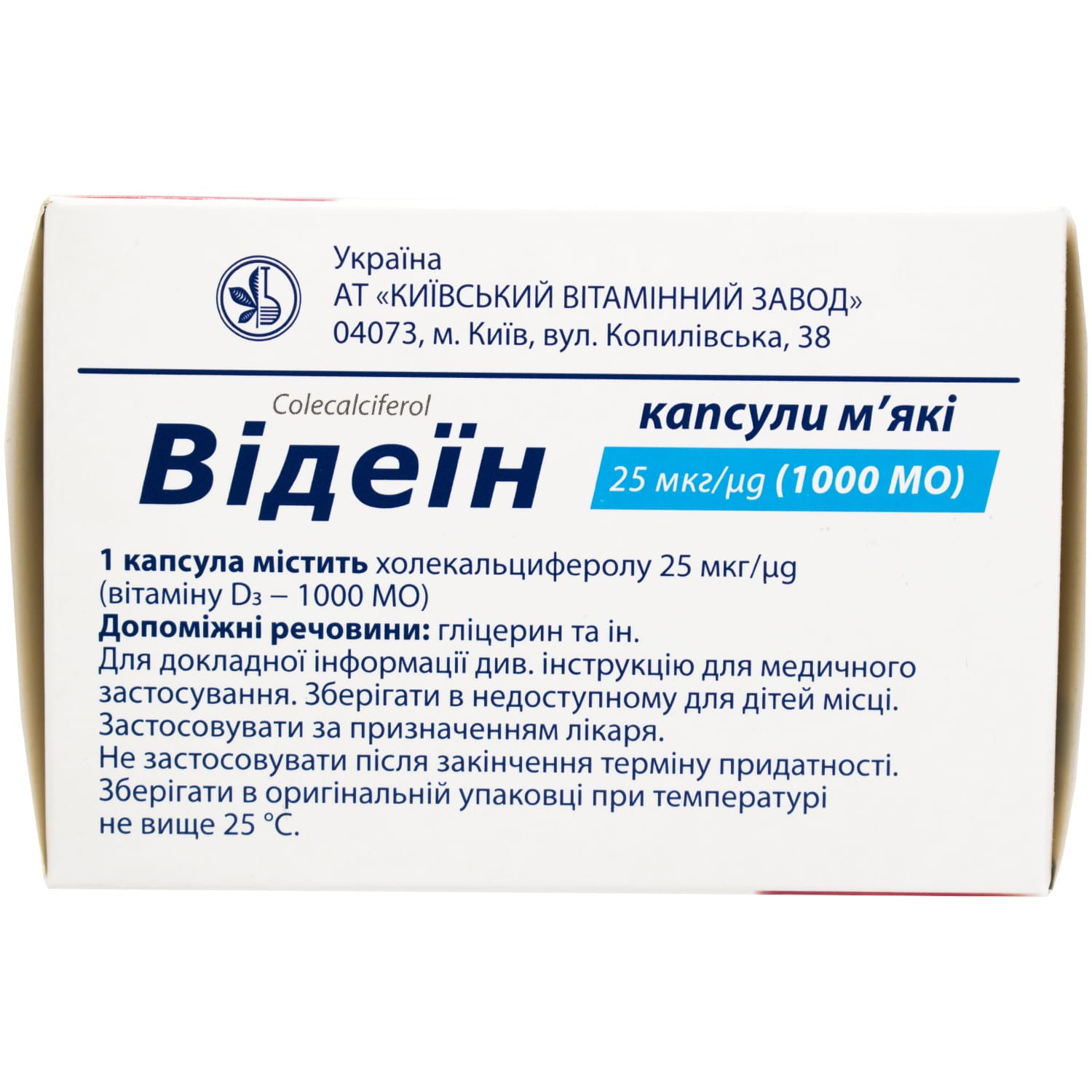
Active substance – cholecalciferol (one capsule contains cholecalciferol 25 µg (vitamin D3 – 1000 IU)).
Excipients: α-tocopherol acetate, medium-chain triglycerides.
Capsule shell: gelatin, glycerol.
Contraindications
Hypersensitivity to the components of the medicinal product, hypercalcemia and/or hypercalciuria, idiopathic hypercalcemia of newborns, hypervitaminosis D, sarcoidosis, renal failure, urolithiasis, nephrolithiasis, tuberculosis.
Pseudohypoparathyroidism (the need for vitamin D may be lower than during periods of normal sensitivity to vitamin D).
Method of administration
The medicinal product should be taken orally.
Adults and older children (if swallowing the capsule is possible) should take the product before or during meals; swallow the capsule whole with a sufficient amount of water.
Children from the second week of life, or adults and older children (if swallowing is not possible), should squeeze the contents of the capsule into a spoon (container) with liquid (milk, fruit juice) or other food products.
If the contents of the capsule are added to a feeding bottle or plate, it is necessary to ensure complete consumption of the food; otherwise, intake of the full dose of the medicinal product cannot be guaranteed. The medicinal product should be added to food immediately before consumption.
Administration scheme (with the possibility of releasing liquid from the capsule)
Squeeze the contents of the capsule into a previously prepared clean spoon (container). To remove the contents of the capsule into a clean spoon (container) for collecting the liquid contents, hands must be thoroughly washed and dried.
With two fingers of one hand, hold the capsule by the tail (for “fish”) or the neck (for “bottles”) and press so that the contents move into the body of the capsule.
With two fingers of the other hand, slightly stretch and tightly pinch the neck of the capsule. Slowly twist the tail or neck until separation. Monitor the neck so that when twisting off the tail or neck the contents do not splash out.
After depressurization of the capsule, squeeze the contents as much as possible into the previously prepared clean spoon (container).
Recommended dosages
Prevention of rickets: the recommended dose is one capsule of 12.5 µg (500 IU of vitamin D3) per day.
Prevention of rickets in premature newborn infants: the dose is determined by the physician. The total recommended dose is one capsule of 25 µg (1000 IU of vitamin D3) per day.
Prevention of vitamin D3 deficiency in high-risk patients without malabsorption disorders: the recommended dose is one capsule of 12.5 µg (500 IU of vitamin D3) per day.
Prevention of vitamin D3 deficiency in malabsorption: the dose is determined individually by the physician. The total recommended dose is 3–5 capsules of 25 µg (3000–5000 IU of vitamin D3) per day.
Treatment of rickets and osteomalacia: the dose is determined individually by the physician depending on the course and severity of the disease. The total recommended dose for the treatment of vitamin D3 deficiency in infants and children is 1–5 capsules of 25 µg (1000–5000 IU of vitamin D3) per day.
Treatment lasts for one year.
Maintenance therapy of osteoporosis: the recommended dose is one capsule of 25 µg (1000 IU of vitamin D3) per day.
Duration of use
The duration of treatment depends on the course and severity of the disease and is determined individually by the physician.
Treatment of rickets and osteomalacia caused by vitamin D3 deficiency lasts for one year.
Children are prescribed the product for the prevention of rickets starting from the second week of life until the end of the first year of life. During the second year of life, further use of the product may be necessary, especially in winter.
When using doses exceeding 1000 IU of vitamin D3 per day, as well as during continuous or long-term treatment, serum creatinine levels and calcium levels in serum and urine should be regularly monitored. If necessary, the dose should be adjusted depending on the serum calcium concentration.
Special precautions for use
Pregnant women
The medicinal product should be used with caution in pregnant and breastfeeding women.
Children
The product is used in children from the second week of life.
Drivers
There are no reports that the medicinal product affects the ability to drive vehicles or operate other mechanisms. However, when driving vehicles or operating other mechanisms, special caution is recommended, taking into account the possibility of adverse reactions from the nervous system.
Overdose
Vitamin D3 regulates calcium and phosphate metabolism; after overdose, hypercalcemia, hypercalciuria, renal calcifications and bone damage occur, as well as changes in the cardiovascular system. Hypercalcemia occurs after the use of 50,000–100,000 IU of vitamin D3 per day.
In case of overdose, the following effects may develop: muscle weakness, loss of appetite, nausea, vomiting, constipation, polydipsia, polyuria, drowsiness, photosensitivity, pancreatitis, rhinorrhea, hyperthermia, decreased libido, conjunctivitis, hypercholesterolemia, increased transaminase activity, arterial hypertension, cardiac arrhythmia and uremia. Common symptoms include muscle and joint pain, headache, weight loss. Renal dysfunction develops with albuminuria, erythrocyturia and polyuria, increased potassium loss, hyposthenuria, nocturia and a moderate increase in blood pressure.
In severe cases, corneal clouding is possible; less often, papilledema, inflammation of the iris up to the development of cataract.
Concretions may form in the kidneys; calcification in soft tissues such as blood vessels, heart, lungs and skin.
Cholestatic jaundice rarely develops.
Treatment
Overdose requires treatment of hypercalcemia. It is necessary to discontinue the medicinal product. Depending on the degree of hypercalcemia, a low-calcium or calcium-free diet, intake of large amounts of fluids, forced diuresis induced by furosemide, as well as administration of glucocorticoids and calcitonin are recommended.
With normal renal function, calcium levels are reliably reduced by infusion of sodium chloride solution (3–6 liters over 24 hours) with the addition of furosemide; in some cases, sodium edetate 15 mg/kg body weight/hour should also be used, with continuous monitoring of calcium levels and ECG. In oligoanuria, on the contrary, hemodialysis is necessary. There is no specific antidote.
Adverse reactions
Adverse reactions are usually not observed when used at recommended doses.
In case of individual hypersensitivity to the product, which is rare, or as a result of using very high doses for a long period, hypervitaminosis D may occur.
From the cardiovascular system: arrhythmia, arterial hypertension.
Gastrointestinal tract: loss of appetite, nausea, vomiting, constipation, dry mouth, flatulence, abdominal pain, diarrhea, dyspepsia, pancreatitis.
From the nervous system: headache, drowsiness, mental disorders, depression.
From the urinary system: increased calcium levels in blood and/or urine, urolithiasis and tissue calcification, uremia, polyuria.
From the skin: hypersensitivity reactions, including urticaria, rash, itching.
From the musculoskeletal system: myalgia, arthralgia, muscle weakness.
From the organs of vision: conjunctivitis, photosensitivity.
From metabolism: hypercholesterolemia, weight loss, polydipsia, increased sweating.
From the hepatobiliary system: increased aminotransferase activity.
From the psyche: decreased libido.
There have also been reports of rhinorrhea, hyperthermia and dry mouth.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C, out of the reach of children.
Shelf life – 2 years.
Be the first to review “Videin soft capsules 25 mcg (1000 IU), 60 capsules” Cancel reply
You may also like







Reviews
There are no reviews yet.