No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
[category_image]
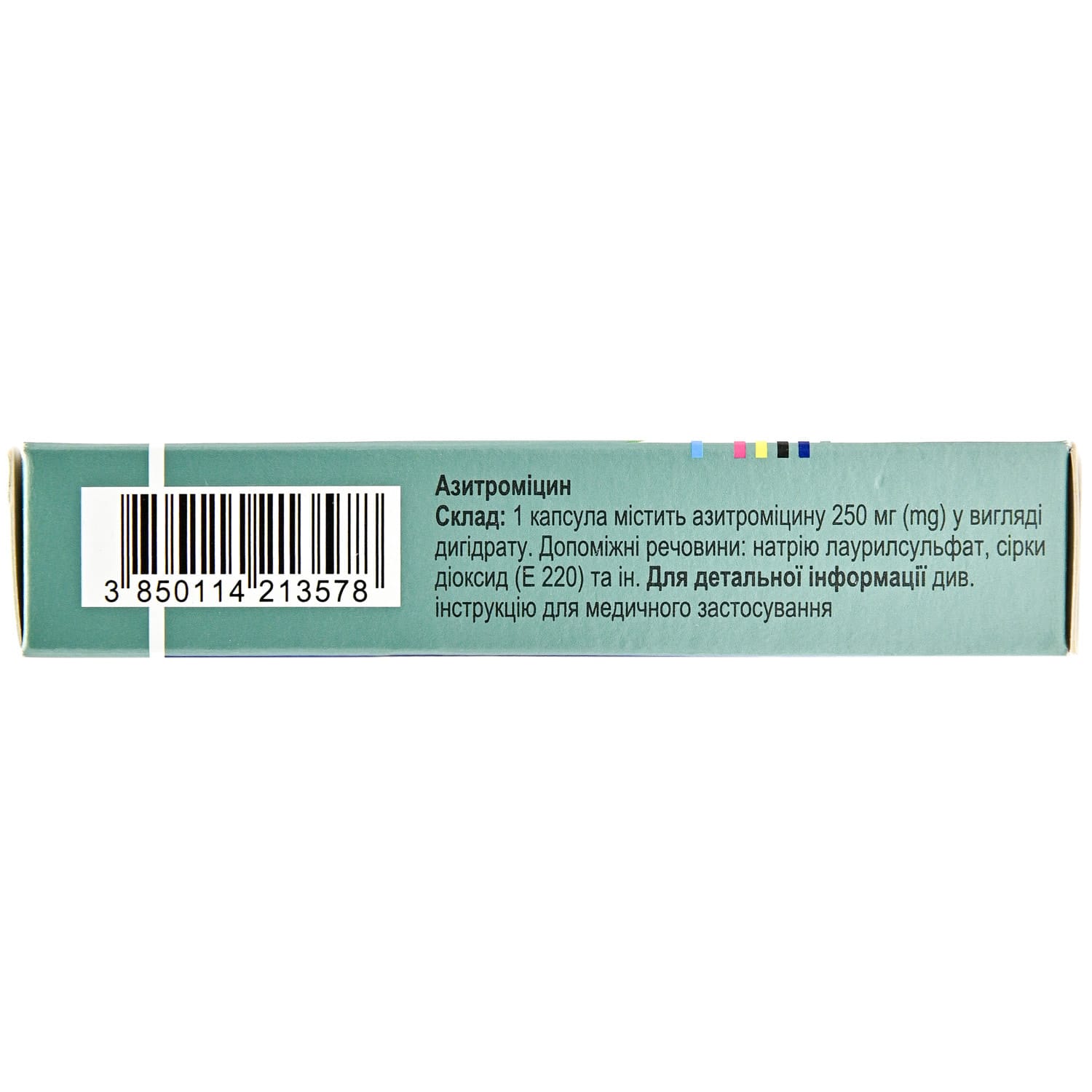
Sumamed capsules 250 mg blister 6 pcs.
$28.58
Sumamed is a macrolide antibiotic with azithromycin, used to treat ENT, respiratory, skin, soft tissue and chlamydial infections.
Categories: Antimicrobials
Brand: Teva
The active substance of Sumamed is the macrolide antibiotic Azithromycin, which belongs to the group of azalides. The molecule is formed by the introduction of a nitrogen atom into the lactone ring of erythromycin A. The mechanism of action of Azithromycin is to suppress bacterial protein synthesis by binding to the 50S subunit of ribosomes and inhibiting peptide translocation.
Indications for use
Infections caused by microorganisms sensitive to azithromycin:
- ENT organs (bacterial pharyngitis/tonsillitis, sinusitis, otitis media);
- respiratory tract infections (bacterial bronchitis, community-acquired pneumonia);
- Skin and soft tissue infections: erythema migrans (initial stage of Lyme disease), erysipelas, impetigo, secondary pyodermatosis, acne vulgaris (common acne) of moderate severity;
- sexually transmitted infections: uncomplicated genital infections caused by Chlamydia trachomatis.
Contraindication
Hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide antibiotic, or to any other component of the drug.
Method of administration and doses
Administer orally 1 hour before or 2 hours after meals. If a dose is missed, the missed dose should be taken as soon as possible, and subsequent doses should be taken at 24-hour intervals.
The dispersible tablet is pre-dissolved in at least 50 ml of water. Stir the resulting suspension thoroughly before use.
Adults and children over 12 years of age with a body weight ≥45 kg.
For infections of the ENT organs, respiratory tract, skin and soft tissues (except chronic migratory erythema), the total dose of azithromycin is 1500 mg (500 mg 1 time per day). The duration of treatment is 3 days.
For erythema migrans, the total dose of azithromycin is 3 g, which should be taken according to the following scheme: 1000 mg on the first day, then 500 mg once a day from the 2nd to the 5th day.
For sexually transmitted infections, the recommended dose of azithromycin is 1000 mg once.
Children 3-12 years old with a body weight <45 kg.
For infections of the ENT organs, respiratory tract, skin and soft tissues (except chronic migratory erythema), azithromycin is used at the rate of 10 mg/kg of body weight once a day for 3 days (the total dose of azithromycin is 30 mg/kg).
Overdose
Clinical experience with azithromycin suggests that the side effects that develop when taking higher than recommended doses of the drug are similar to those observed with the use of conventional therapeutic doses. They may include diarrhea, nausea, vomiting, reversible hearing loss. In case of overdose, if necessary, it is recommended to take activated charcoal and carry out general symptomatic and supportive measures.
Special instructions
Use during pregnancy or breastfeeding.
Pregnancy.
There are no adequate data on the use of azithromycin in pregnant women. In animal reproductive toxicity studies, azithromycin did not show teratogenic effects on the fetus, but the drug crossed the placenta. The safety of azithromycin during pregnancy has not been confirmed. Therefore, azithromycin should be used during pregnancy only if the benefit outweighs the risk.
Breastfeeding.
Azithromycin has been reported to pass into breast milk, but adequate and well-controlled clinical studies have not been conducted to characterize the pharmacokinetics of azithromycin excretion into breast milk.
Fertility.
Fertility studies have been performed in rats; pregnancy rates were reduced following administration of azithromycin. The relevance of these findings to humans is unknown.
Children
Used for children aged 3 years and over.
The ability to influence the reaction speed when driving vehicles or other mechanisms.
There is no evidence that azithromycin can impair the ability to drive or use other mechanisms, but the possibility of developing adverse reactions such as delirium, hallucinations, dizziness, drowsiness, fainting, and seizures, which may affect the ability to drive or use other mechanisms, should be taken into account.
Composition
active ingredient: azithromycin;
1 tablet contains 250 mg of azithromycin in the form of azithromycin dihydrate;
Excipients: saccharin sodium dihydrate, microcrystalline cellulose, crospovidone (type A), povidone, sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate, aspartame (E 951), 125 mg tablets – banana flavoring, orange flavoring.
Storage conditions
The drug does not require special storage conditions. Keep out of the reach of children.
Shelf life – 3 years.
Be the first to review “Sumamed capsules 250 mg blister 6 pcs.” Cancel reply
You may also like









Reviews
There are no reviews yet.