No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
[category_image]
Sumamed powder for oral suspension with strawberry flavor 100 mg/5 ml bottle 20 ml (400 mg) 1 pc.
$26.58
Strawberry-flavored Sumamed oral suspension treats ENT, respiratory, skin and soft tissue infections in children with azithromycin once daily.
Categories: Antimicrobials
Brand: Teva
Powder for oral suspension with strawberry flavor Sumamed is used for infections caused by microorganisms sensitive to azithromycin:
- ENT organs (bacterial pharyngitis/tonsillitis, sinusitis, otitis media);
- respiratory tract infections (bacterial bronchitis, community-acquired pneumonia);
- Skin and soft tissue infections: erythema migrans (initial stage of Lyme disease), erysipelas, impetigo, secondary pyodermatoses.
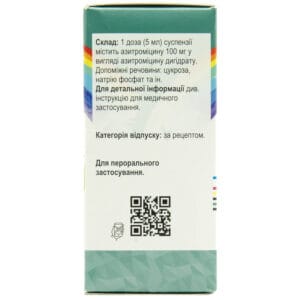
Composition of Sumamed
Active ingredient: azithromycin;
1 dose (5 ml) of suspension contains 100 mg of azithromycin as azithromycin dihydrate;
Excipients: sucrose, sodium phosphate, hydroxypropylcellulose, xanthan gum, colloidal silicon dioxide;
Flavoring(s) and/or coloring: banana flavoring, cherry flavoring, vanilla flavoring or titanium dioxide (E 171), strawberry flavoring.
Contraindication
Hypersensitivity to azithromycin, erythromycin or any macrolide or ketolide antibiotic, or to any other component of the drug.
Method of application
Powder for oral suspension, used once daily at least 1 hour before or 2 hours after a meal.
If you miss a dose of the drug, take the missed dose as soon as possible, and the following doses should be taken at 24-hour intervals.
Application features
Pregnant women
Azithromycin is prescribed during pregnancy only if the benefit outweighs the risk.
Azithromycin has been reported to pass into breast milk, but adequate and well-controlled clinical studies have not been conducted to characterize the pharmacokinetics of azithromycin excretion into breast milk.
Fertility studies have been conducted in rats; pregnancy rates were reduced following administration of azithromycin. The relevance of these findings to humans is unknown.
Children
Use in children weighing 5 to 15 kg.
Drivers
There is no evidence that azithromycin can impair the ability to drive or operate complex mechanisms, but the possibility of developing adverse reactions such as delirium, hallucinations, dizziness, drowsiness, fainting, and seizures, which may affect the ability to drive or operate machinery, should be taken into account.
Overdose
Experience with the clinical use of azithromycin suggests that the side effects that develop when taking higher than recommended doses of the drug are similar to those observed when using conventional therapeutic doses, namely: they may include diarrhea, nausea, vomiting, reversible hearing loss. In case of overdose, if necessary, it is recommended to take activated charcoal and carry out general symptomatic and supportive treatment measures.
Side effects
From the nervous system: often (≥ 1/100 to <1/10) – headache.
Gastrointestinal tract: very often (≥ 1/10) – diarrhea; often (≥ 1/100 to <1/10) – vomiting, abdominal pain, nausea.
Interaction
Antacids: In a study of the effect of concomitant administration of antacids on the pharmacokinetics of azithromycin, there was generally no change in bioavailability, although the maximum plasma concentration of azithromycin was reduced by approximately 25%. Azithromycin and antacids should not be taken simultaneously.
Cetirizine: In healthy volunteers, coadministration of azithromycin for 5 days with cetirizine 20 mg at steady state did not result in any pharmacokinetic interaction or significant changes in the QT interval.
Didanosine: Coadministration of daily doses of 1200 mg azithromycin with 400 mg didanosine per day in six HIV-positive volunteers had no effect on the steady-state pharmacokinetics of didanosine compared to placebo.
Storage conditions of Sumamed
Store at a temperature not exceeding 25 °C, out of the reach of children.
Store the prepared suspension at a temperature not exceeding 25 °C.
Shelf life – 2 years.
The shelf life of the finished suspension is 5 days.
Be the first to review “Sumamed powder for oral suspension with strawberry flavor 100 mg/5 ml bottle 20 ml (400 mg) 1 pc.” Cancel reply
You may also like









Reviews
There are no reviews yet.