No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
[category_image]
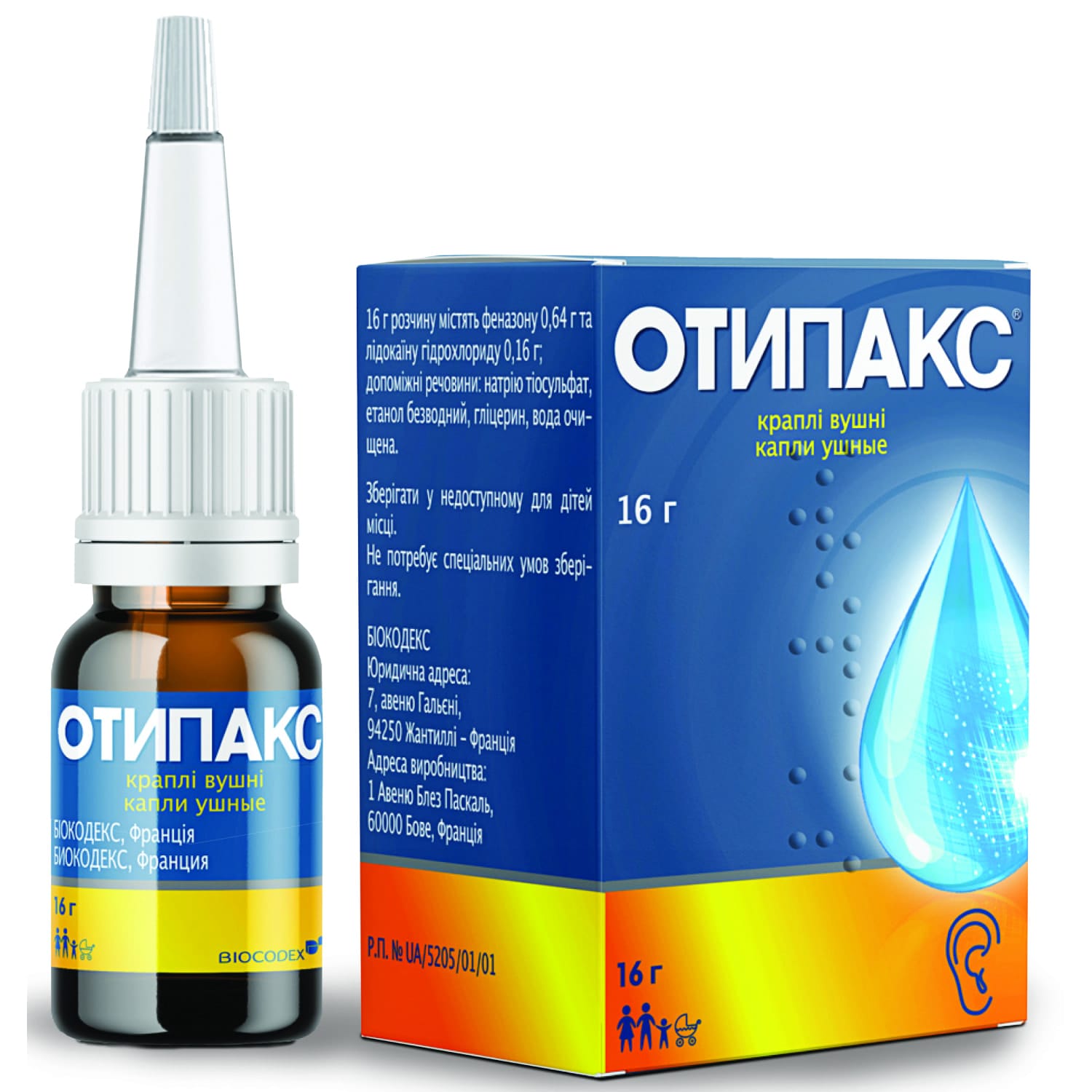
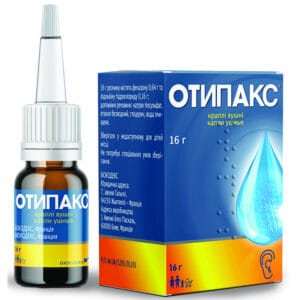
Otipax ear drops 16 g bottle with plastic dropper
$22.52
Otipax ear drops with phenazone and lidocaine relieve ear pain and inflammation in adults and children with otitis and barotrauma safely and fast acting
Categories: Eye and ear
Brand: Biocodex
Pharmacological properties
Pharmacodynamics. Otipax is a combination of two active ingredients: phenazone and lidocaine. Phenazone is a pyrazolone derivative with analgesic and anti-inflammatory properties. Lidocaine is a local anesthetic of the amide group. The combination of phenazone with lidocaine causes a pronounced analgesic / anti-inflammatory effect.
Pharmacokinetics. The absorption of any component of the drug through the skin has not been studied. Systemic absorption of the solution is not expected (in the absence of damage to the eardrum).
The drug’s effect (reducing eardrum pain and reducing inflammation) begins 5 minutes after instillation. The pain syndrome almost completely disappears after 15-30 minutes.
Indication
Local symptomatic treatment of certain painful conditions of the middle ear with an intact tympanic membrane in children aged 1 month and older and adults with:
- otitis media in the acute period;
- phlyctenulosis viral otitis (post-influenza);
- barotraumatic otitis.
Application
Children aged 1 month and older and adults should instill 4 drops 2-3 times a day into the external auditory canal of the ear in which pain is felt. The course of treatment should not exceed 10 days. After that, treatment should be reviewed.
Method of application: For use in the ear.
To prevent discomfort resulting from contact of the skin of the ear canal with a cold solution, warm the bottle in your hands before using the medicine. Unscrew the bottle cap. Remove the dropper from the polyethylene/paper blister. Screw the dropper onto the bottle. Remove the cap from the dropper. Turn the bottle upside down, gently press the dropper to obtain 1 drop. Press further until 4 drops are obtained. Close the dropper with the white cap.
Contraindication
Hypersensitivity to the active substances, to any of the components of the drug or to amide local anesthetics. Perforation of the eardrum of traumatic or infectious origin (see special instructions).
Side effects
Reported adverse reactions are listed according to system organ class.
From the organs of hearing and balance: local reactions: allergic reactions, including irritation, hyperemia of the external auditory canal, itching, skin rashes.
Special instructions
Before any use of the drug, the integrity of the eardrum should be checked (as a precaution). If there is destruction of the eardrum, the introduction of the drug into the ear may lead to contact of the drug with the structures of the middle ear, causing adverse reactions in these tissues.
It should be noted that the drug contains an active ingredient that may show a positive result in an anti-doping test.
Use during pregnancy and breastfeeding. No effects are expected from the use of the drug during pregnancy, since the systemic exposure to phenazone and lidocaine is insignificant.
Under normal conditions of use, phenazone and lidocaine do not penetrate into breast milk.
If necessary, Otipax can be used during pregnancy and breastfeeding after consulting a doctor.
Children. There are no data on the safety and efficacy of Otipax in children under 1 month of age.
Use in children aged 1 month and over after consultation and on the recommendation of a doctor.
Ability to influence the reaction rate when driving vehicles or other mechanisms. The drug does not affect the ability to drive vehicles and operate potentially dangerous mechanisms.
Interactions
There is currently no evidence of clinically significant interactions.
Overdose
When using the drug in the recommended dosage, no overdose was observed.
Storage conditions
No special storage conditions are required. Keep out of the reach of children.
You may also like