🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
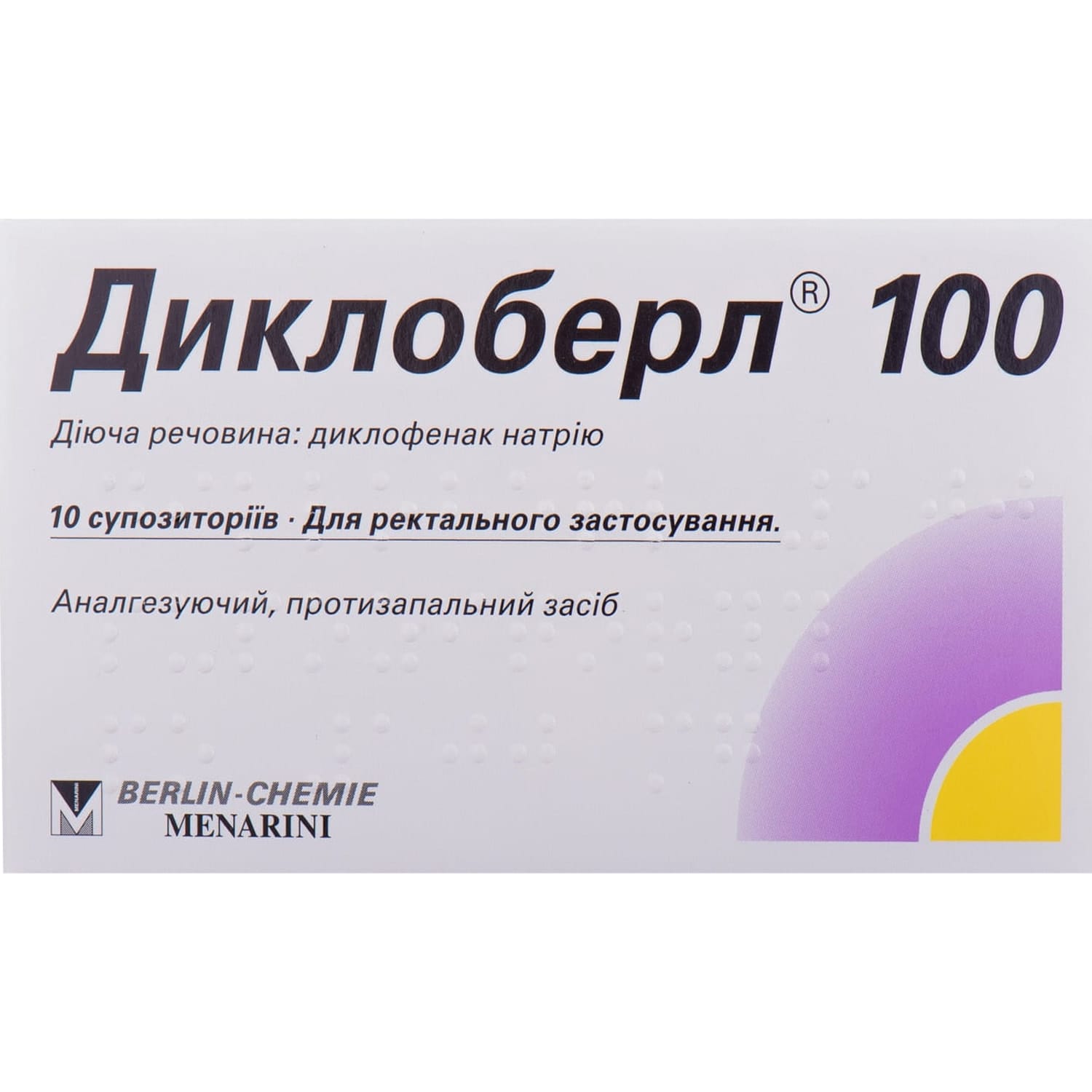
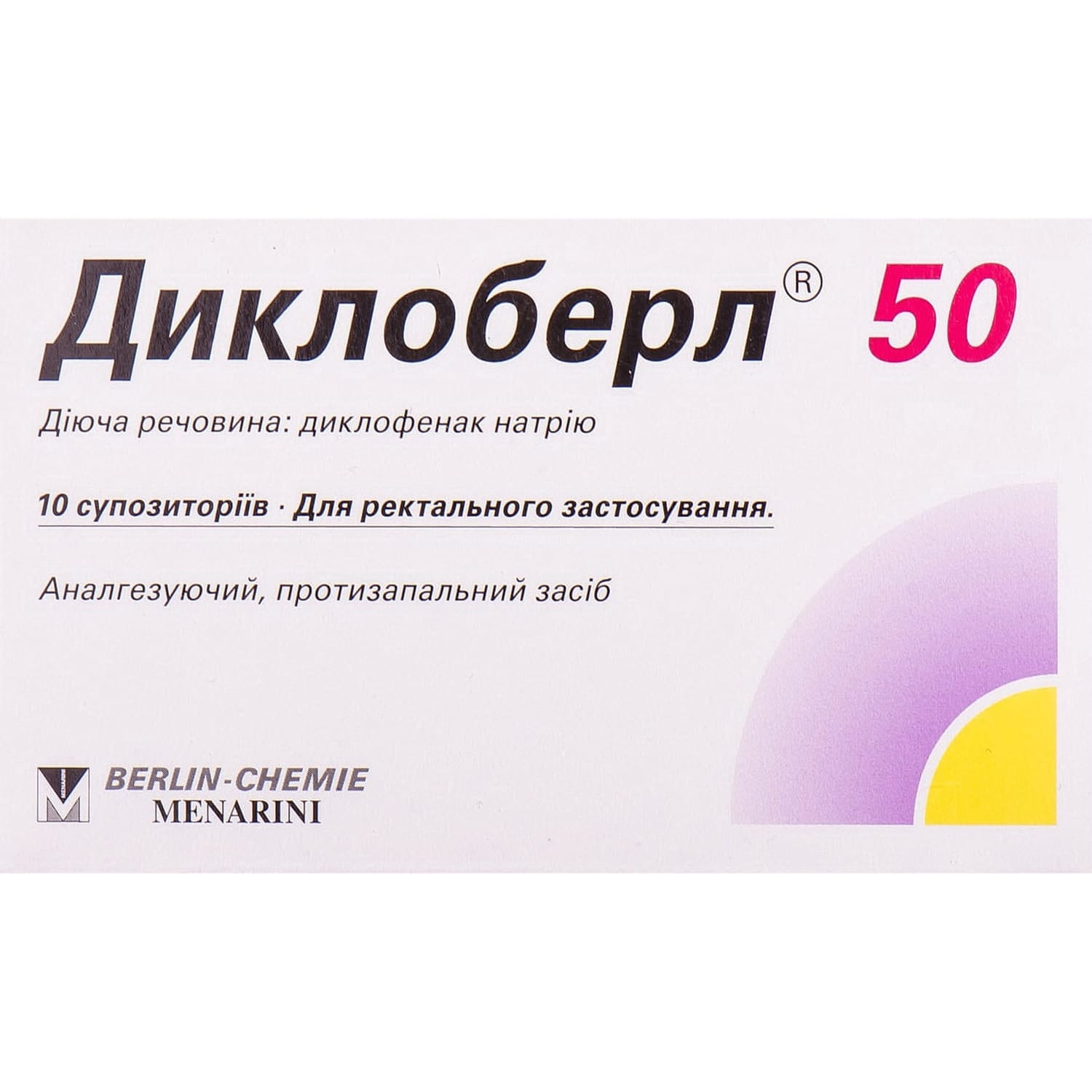
Dicloberl 50 suppositories (candles) 50 mg each 10 pcs.
$16.66
Dicloberl 50 suppositories with diclofenac relieve pain and inflammation in rheumatic, gynecologic, postoperative conditions, migraine, gout, and ENT pain.
Dicloberl 50 is a nonsteroidal anti-inflammatory and antirheumatic agent.
Indications for use
- inflammatory and degenerative forms of rheumatism: rheumatoid arthritis, juvenile rheumatoid arthritis, ankylosing spondylitis, osteoarthritis, including spondyloarthritis;
- pain syndromes in the spine;
- rheumatic diseases of extra-articular soft tissues;
- post-traumatic and postoperative pain syndromes accompanied by inflammation and swelling, in particular after dental and orthopedic surgeries;
- gynecological diseases accompanied by pain and inflammation, such as primary dysmenorrhea and adnexitis;
- migraine attacks;
- acute attacks of gout;
- as an adjuvant for severe inflammatory diseases of the ENT organs, which are accompanied by pain, for example, with pharyngotonsillitis, otitis.
According to general therapeutic principles, the underlying disease should be treated with basic therapy. Fever alone is not an indication for the use of the drug.
Composition
- active ingredient: diclofenac sodium;
- 1 suppository contains diclofenac sodium 50 mg;
- excipients: propyl gallate, ethanol 96%, corn starch, solid fat.
Contraindication
- hypersensitivity to the active substance or to any excipient of the medicinal product;
- Dicloberl® 50, like other nonsteroidal anti-inflammatory drugs (NSAIDs), is contraindicated in patients who develop attacks of bronchial asthma, urticaria, angioedema, acute rhinitis or nasal polyps in response to taking acetylsalicylic acid or other NSAIDs;
- unspecified hematopoietic disorders;
- active ulcer/bleeding or history of recurrent ulcer/bleeding (two or more separate episodes of diagnosed ulcer or bleeding);
- history of gastrointestinal bleeding or perforation related to previous NSAID treatment;
- inflammatory bowel disease (e.g. Crohn’s disease or ulcerative colitis);
- acute stomach or intestinal ulcer, bleeding or perforation;
- cerebrovascular or other active bleeding;
- severe liver or kidney dysfunction, liver failure, renal failure (glomerular filtration rate <15 ml/min/1.73 m2);
- treatment of perioperative pain during coronary artery bypass grafting (or the use of a cardiopulmonary bypass machine);
- congestive heart failure (NYHA II-IV); ischemic heart disease in patients with angina pectoris, myocardial infarction; peripheral arterial disease and/or cerebrovascular disease in patients with stroke or transient ischemic attacks;
- the last trimester of pregnancy;
- proctitis.
Adverse reactions
The most common adverse reactions are gastrointestinal.
Peptic ulcer, perforation or GI bleeding, sometimes fatal, especially in the elderly, may occur (see section 4.4). Nausea, vomiting, diarrhoea, bloating, constipation, dyspepsia, abdominal pain, melena, haematemesis, gastritis, ulcerative stomatitis, exacerbation of ulcerative colitis and Crohn’s disease have been reported following the use of the drug.
In particular, the risk of SC bleeding depends on the dose and duration of use.
Edema, hypertension, and heart failure have been reported in association with the use of NSAIDs.
Clinical trials and epidemiological data consistently indicate an increased risk of arterial thrombotic events (e.g. myocardial infarction or stroke) associated with the use of diclofenac, especially at high doses (150 mg per day) and with long-term treatment.
Method of application
Undesirable effects can be minimized by using the lowest effective dose for the shortest period of time necessary to control symptoms.
Do not use internally, for rectal administration only.
Suppositories should be inserted into the rectum as deeply as possible, preferably after bowel cleansing.
The initial dose is usually 100-150 mg per day. With mild symptoms, as well as with long-term therapy, a dose of 75-100 mg per day is sufficient.
Divide the daily dose into 2-3 doses. To avoid night pain or morning stiffness, administer Dicloberl 50 in the form of rectal suppositories before bedtime (the daily dose of the drug should not exceed 150 mg).
In primary dysmenorrhea, the daily dose is selected individually, usually it is 50-150 mg per day. The initial dose may be 50-100 mg per day, but if necessary it can be increased over several menstrual cycles to a maximum of 150 mg per day. The drug should be started after the first painful symptoms appear and continued for several days, depending on the dynamics of symptom regression.
For the treatment of migraine attacks, the course should be started at a dose of 100 mg at the first signs of the onset of an attack. If necessary, the following suppositories (100 mg of diclofenac) can be used on the same day. If necessary, the treatment can be continued on the following days (the daily dose of the drug should not exceed 150 mg, the dose should be divided into 2-3 applications).
In the treatment of juvenile rheumatoid arthritis, the daily dose may be set at up to 3 mg/kg, which is the maximum daily dose and should not exceed 150 mg per day. Children aged 14 years and older may be given 50 mg suppositories.
Application features
Use during pregnancy or breastfeeding
During the first and second trimesters of pregnancy, diclofenac should not be used unless clearly necessary.
Dicloberl 50 is contraindicated during the third trimester of pregnancy (see section “Contraindications”).
Like other NSAIDs, diclofenac passes into breast milk in small amounts. Therefore, Dicloberl® 50 should not be used by women during breastfeeding to avoid undesirable effects on the infant. If treatment is life-threatening, the child should be transferred to artificial feeding.
Children
Dicloberl 50 should not be used in children under 14 years of age due to its high content of active ingredient. The drug can be used in children over 14 years of age.
Ability to influence reaction speed when driving vehicles or other mechanisms
Since the use of Dicloberl® in high doses may cause undesirable effects on the central nervous system, such as fatigue and dizziness, the ability to react and the ability to actively participate in traffic and operate machinery may be impaired in individual cases. This is especially true when the drug is used in combination with alcohol. Patients who experience such effects should refrain from driving or using machinery.
Overdose
Symptoms of overdose
There is no typical clinical picture characteristic of diclofenac overdose. Central nervous system disorders such as headache, dizziness, vertigo, agitation, coma, drowsiness, tinnitus and loss of consciousness (also myoclonic convulsions in children), as well as abdominal pain, diarrhea, nausea and vomiting, may occur as symptoms of overdose. In addition, gastrointestinal bleeding is possible, as are impaired liver and kidney function, acute renal failure and liver damage are possible in case of severe intoxication. There may also be arterial hypotension, respiratory depression and cyanosis.
Therapeutic measures in case of overdose
There is no specific antidote.
Management of acute poisoning with NSAIDs, including diclofenac, consists essentially of supportive measures and symptomatic treatment. For complications such as hypotension, renal failure, convulsions, gastrointestinal disorders and respiratory depression, supportive measures and symptomatic treatment should be used.
Special measures such as forced diuresis, dialysis or haemoperfusion are unlikely to be helpful in eliminating NSAIDs, including diclofenac, due to high protein binding and active metabolism. The use of activated charcoal should be considered within one hour of ingestion of a potentially toxic amount of the drug. In addition, gastric lavage should be considered in adults within one hour of ingestion of a potentially toxic amount of the drug. In cases of frequent or prolonged convulsions, intravenous diazepam should be administered. Other measures may be indicated depending on the clinical condition of the patient.
Storage conditions
Store at a temperature not exceeding 25 ° C. Keep the medicine out of the reach of children.
You may also like