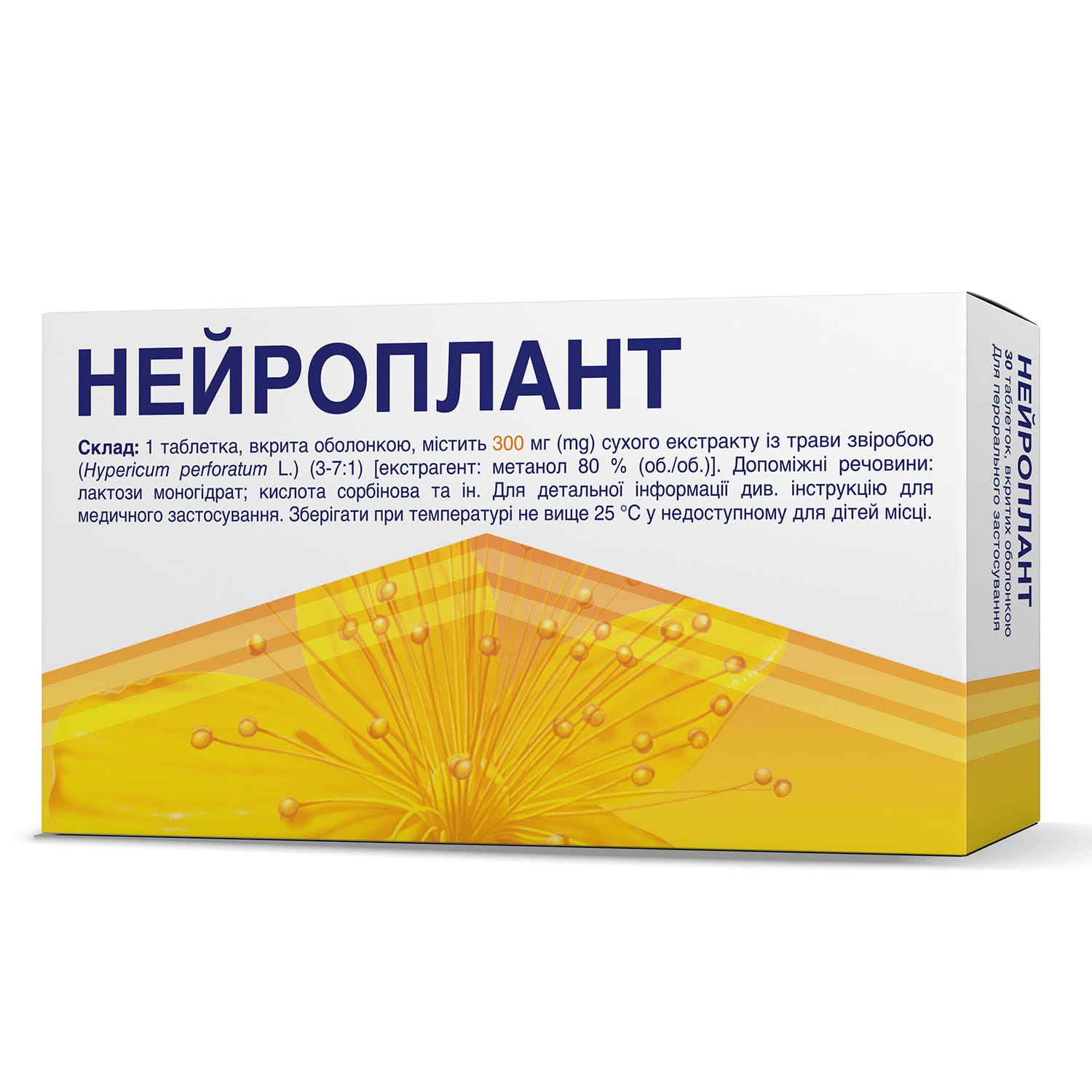
Neuroplant – St. John’s wort for mild depression. Adults: 300 mg 3×/day. May cause photosensitivity or allergies. Not for children or pregnancy.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more

Amixin IC film-coated tablets ...
$24.39 Original price was: $24.39.$22.19Current price is: $22.19.
Neuroplant – an antidepressant
Indications for use
Treatment of mild depressive episodes.
Composition
Active substance: dry extract of St. John’s wort herb (Hypericum perforatum L.)
1 film-coated tablet contains 300 mg of dry extract of St. John’s wort herb (Hypericum perforatum L.) (3–7:1) [extraction solvent: methanol 80% (v/v)].
Excipients: ascorbic acid, microcrystalline cellulose, croscarmellose sodium, hypromellose, lactose monohydrate, macrogol 4000, magnesium stearate, sodium saccharin, silicon dioxide, talc, vanillin, titanium dioxide (E171), yellow iron oxide (E172), pregelatinized starch, antifoam emulsion (contains methylcellulose, simethicone, sorbic acid).
Contraindications
Hypersensitivity to any component of the medicinal product. Known photosensitivity of the skin, photosensitization. Concomitant treatment with medicinal products metabolized by cytochrome P450 3A4, CYP2C9, CYP2C19 and P-glycoproteins:
- protease inhibitors for the treatment of HIV infection (cyclosporine, tacrolimus, indinavir or others);
- protease inhibitors of the hepatitis C virus;
- immunosuppressants;
- cytostatic agents used in cancer treatment (irinotecan, imatinib and others);
- amitriptyline, nortriptyline;
- midazolam;
- theophylline;
- hypocholesterolemic agents (statins);
- cardiac glycosides;
- coumarin anticoagulants (warfarin);
- other antidepressants;
- oral contraceptives, injectable contraceptives and contraceptive implants.
Severe depressive states. Arterial hypertension.
Adverse reactions
Skin and subcutaneous tissue / immune system: allergic reactions (redness, swelling, itching, rash). Photosensitivity: in isolated cases, Neuroplant may cause, especially in people with fair and sensitive skin, reactions similar to sunburn on skin areas exposed to intense sunlight or other types of UV radiation (photosensitization) and dysesthesia (e.g. tingling, sensitivity to cold or pain, burning sensation).
Nervous system: apathy or anxiety.
Gastrointestinal disorders may occur rarely.
If any adverse reactions occur, treatment should be discontinued and a doctor should be consulted.
Method of administration
Adults and elderly patients: single dose – 300 mg.
Frequency of administration – 3 times daily.
Daily dose – 900 mg.
Duration of treatment
To achieve a noticeable improvement in symptoms, treatment for 4 weeks is required. If symptoms persist for more than four weeks or if the condition worsens despite correct use, a doctor should be consulted.
Film-coated tablets should be swallowed whole with a sufficient amount of liquid. Tablet intake does not depend on meals.
Special precautions
Use during pregnancy and breastfeeding
Due to insufficient data, Neuroplant should not be used during pregnancy or breastfeeding.
Children
The medicinal product should not be used in children.
Ability to influence reaction speed when driving or operating machinery
Unknown.
Overdose
After intake of excessive doses of the medicinal product, phototoxic reactions may occur. In this case, the skin should be protected from sunlight and UV radiation for one week (reduce time spent outdoors, wear appropriate clothing and use sunscreens with a high protection factor). Treatment of phototoxic skin reactions is symptomatic.
There have been reports of seizures and confusion after intake of 4.5 g of dry St. John’s wort extract per day for two weeks, as well as an additional intake of 15 g of dry extract. In case of overdose, a bitter taste in the mouth and unpleasant sensations in the liver area may also occur.
Interaction with other medicinal products and other types of interactions
With concomitant use of Neuroplant, the effectiveness of the following medicinal products may be reduced: coumarin anticoagulants (e.g. phenprocoumon, warfarin); amitriptyline, nortriptyline; midazolam; theophylline; antibiotics; sulfonamides; calcium channel blockers; female sex hormones; hypocholesterolemic agents (statins); cardiac glycosides. Therefore, their concomitant use is contraindicated.
With concomitant use of oral contraceptives, intermenstrual bleeding may occur; the reliability of contraceptive methods may be reduced.
With concomitant therapy using medicinal products that increase skin sensitivity to light, an increased sensitivity to sunlight may occur.
Medicinal products containing St. John’s wort extract may reduce plasma concentrations of medicinal products metabolized by cytochrome P450 3A4, CYP2C9, CYP2C19 and P-glycoproteins (e.g. amitriptyline, nortriptyline, fexofenadine, benzodiazepines and their derivatives, methadone, simvastatin, finasteride), antidepressants and other serotonergic substances (such as buspirone, citalopram, escitalopram, fluoxetine, sertraline, triptans, nefazodone, tramadol and others).
Storage conditions
Store at a temperature not exceeding 25 °C, out of reach of children.
You may also like