🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
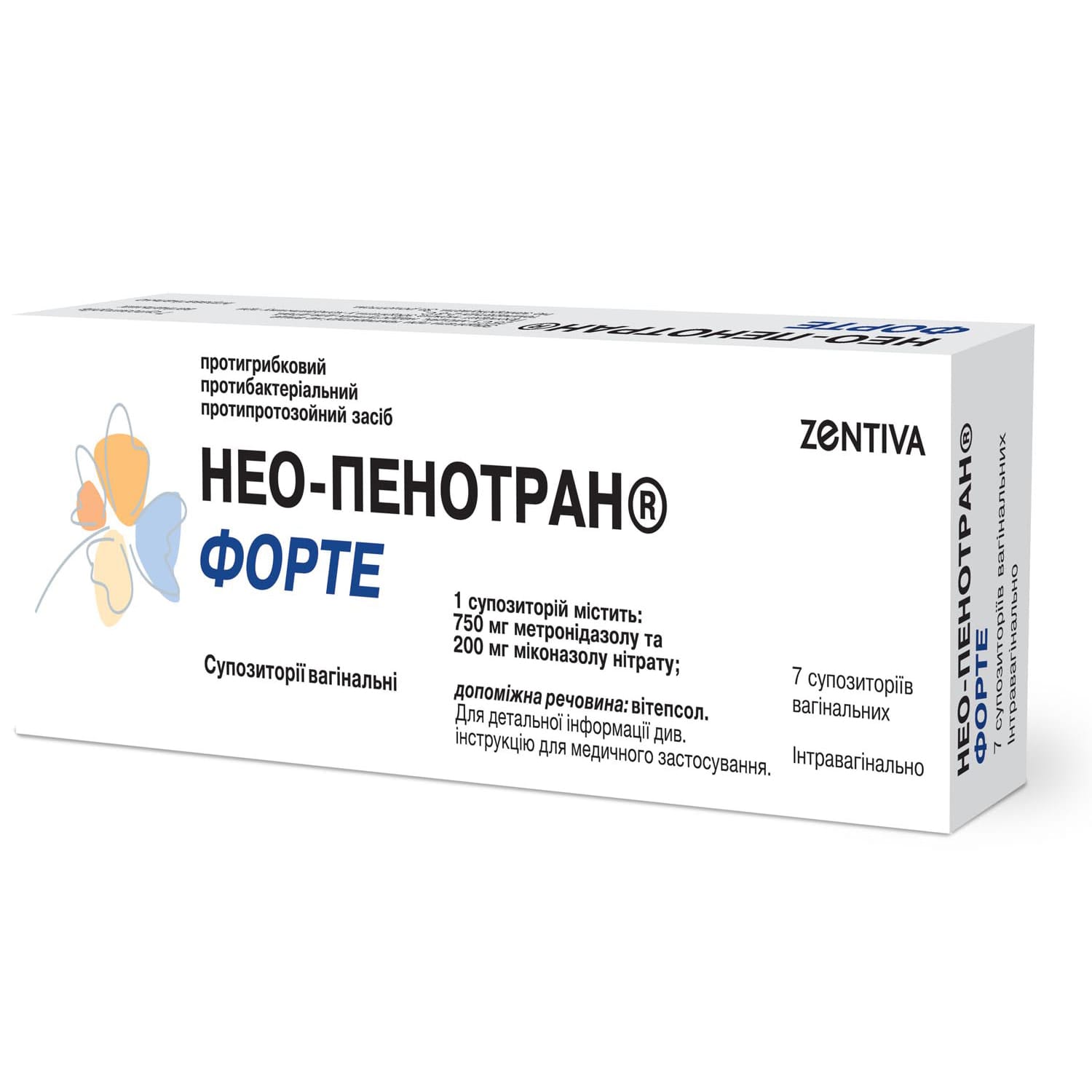
Neo-Penotran Forte vaginal suppositories blister 7 pcs
$33.52
Neo-Penotran – vaginal treatment combining metronidazole and miconazole with antibacterial, antiprotozoal, and antifungal action.
-
— or —
Neo-Penotran Forte vaginal suppositories are used to treat candidal vulvovaginitis caused by Candida albicans, bacterial vaginosis caused by anaerobic bacteria and Gardnerella vaginalis, trichomonas vaginitis caused by Trichomonas vaginalis, and mixed vaginal infections.
Composition
1 suppository contains (active ingredients):
- metronidazole – 750 mg;
- miconazole nitrate – 200 mg.
The excipient is vitepsol.
Contraindication
- hypersensitivity to any of the active ingredients of the drug or to their derivatives;
- drinking alcoholic beverages during treatment or within 3 days after treatment;
- taking disulfiram during treatment or within 2 weeks after treatment;
- porphyria;
- epilepsy;
- severe liver dysfunction.
Method of application
One vaginal suppository should be inserted deep into the vagina at night for 7 days.
For relapses of the disease or vaginitis resistant to other treatments, the drug should be used for 14 days.
It is not recommended to use Neo-Penotran Forte during menstruation due to a decrease in the effectiveness of the drug and the possibility of some complications during administration.
Application features
Pregnant women
Pregnancy – category C.
Since the effects of the drug’s active ingredients on the fetus and neonatal development have not been fully studied, women who need to use this drug should avoid pregnancy by using an effective contraceptive method.
Preclinical animal data on pregnancy, embryonic and fetal development, perinatal and/or postnatal development are insufficient. The potential risk for humans is unknown.
The drug “Neo-Penotran Forte” should not be used in the first trimester of pregnancy. In the second and third trimesters, the drug can be used only if necessary, if the doctor decides that the benefit outweighs the risk.
There is no evidence of a harmful effect on human or animal fertility when metronidazole or miconazole nitrate are used separately.
Breastfeeding should be discontinued during treatment with the drug, as metronidazole, one of the active ingredients of the drug, passes into breast milk. Breastfeeding can be resumed 1-2 days after the end of treatment.
Children
The drug is not recommended for use in children.
Drivers
Systemic use of metronidazole may affect the ability to drive or operate machinery. Compared to systemic use, the absorption of metronidazole is significantly lower with vaginal administration. There is a possibility of dizziness, ataxia, psychoemotional disorders. In the presence of such symptoms, it is not recommended to drive or operate machinery.
Overdose
There are no data on overdose of metronidazole when administered vaginally. When administered into the vagina, metronidazole can be absorbed in an amount sufficient to cause systemic effects.
If a large amount of the drug accidentally enters the digestive system, an appropriate gastric lavage method should be used if necessary. Treatment should be carried out in cases where 12 g of metronidazole has entered the digestive system. There is no specific antidote, symptomatic treatment is recommended. In case of an overdose of metronidazole, the following symptoms are observed: nausea, vomiting, abdominal pain, diarrhea, itching, metallic taste in the mouth, ataxia, vertigo, paresthesia, convulsions, leukopenia, darkening of urine.
In case of an overdose of miconazole nitrate, the following symptoms are observed: nausea, vomiting, inflammation of the throat and oral cavity, anorexia, headache, diarrhea.
Side effects
In isolated cases, side effects such as hypersensitivity reactions (including skin rash) and abdominal pain, headache, itching, burning and irritation of the vagina may occur. The incidence of systemic side effects is very low due to the very low level of metronidazole in the blood plasma when the drug is used vaginally (2-12% of the level achieved with oral metronidazole). Another active ingredient of the drug, miconazole nitrate, can cause vaginal irritation (burning, itching), like all other antifungal agents containing imidazole derivatives administered vaginally (2-6%). In case of severe irritation, treatment should be discontinued.
Storage conditions
Store at a temperature not exceeding 25 °C, out of the reach of children. The drug can be stored in a refrigerator at a temperature of 2-8 °C. Do not freeze.
Shelf life – 3 years.
You may also like







Reviews
There are no reviews yet.