No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
Cinnabsin tablets 60 pcs.
$26.84


Fervex for adults powder for o...
$24.79 Original price was: $24.79.$22.49Current price is: $22.49.
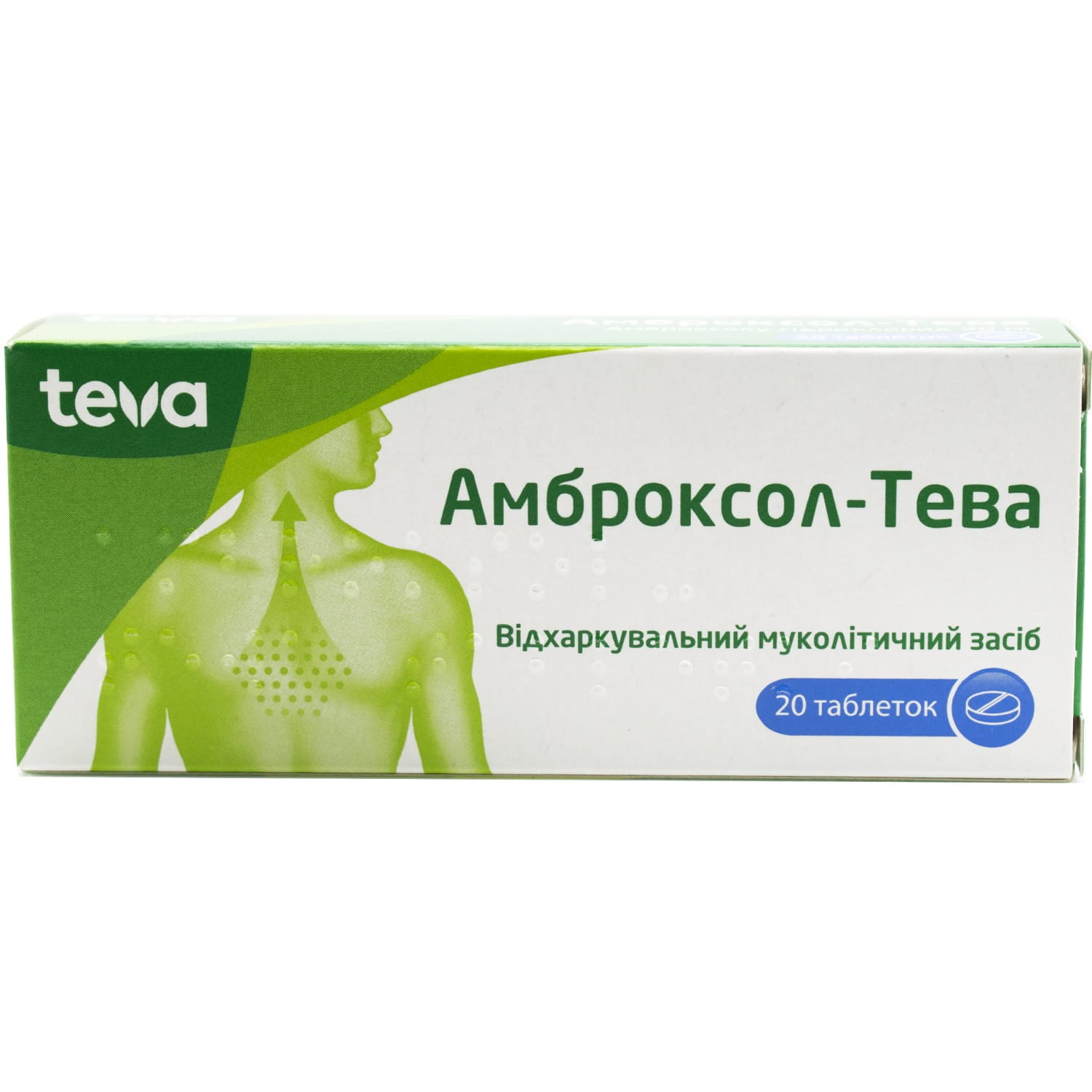
Ambroxol Teva tablets 30 mg 20 pcs.
$15.01
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, South Korea, Switzerland, United Kingdom, United States and more
In stock
Ambroxol Teva 30 mg tablets help thin and remove mucus in acute and chronic bronchopulmonary diseases in adults and children over 12 years.
Categories: Cold and flu
Brand: Teva
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Ambroxol Teva tablets are used in secretolytic therapy for acute and chronic bronchopulmonary diseases associated with impaired bronchial secretion and impaired mucus movement.
Composition
Active ingredient: 1 tablet contains ambroxol hydrochloride – 30 mg;
Excipients: lactose, corn starch, magnesium stearate, silicon dioxide.
Contraindication
- allergy to ambroxol or other components of the drug;
- stomach and duodenal ulcer;
- first trimester of pregnancy;
- breastfeeding period;
- children’s age up to 12 years.
Method of application
30 mg tablets are taken orally after meals with a sufficient amount of warm liquid (for example, tea or broth).
Adults and children over 12 years of age: in the first 3 days, 1 tablet 3 times a day (equivalent to 30 mg of ambroxol hydrochloride 3 times a day). Then – 1 tablet 2 times a day (equivalent to 30 mg of ambroxol hydrochloride 2 times a day).
The duration of treatment depends on the characteristics of the course of the disease. It is not recommended to take 30 mg tablets without a doctor’s prescription for more than 4-5 days.
In case of impaired kidney function and severe liver diseases, the drug can be taken only under the supervision of a doctor. In this case, it is recommended to reduce the dose and increase the time between doses of the drug.
Application features
Pregnant women
It is not recommended to use the drug in the first trimester of pregnancy.
Children
If a child has problems swallowing tablets, it is recommended to use other dosage forms of ambroxol.
Overdose
There are currently no reports of specific symptoms of overdose. The symptoms known from isolated reports of overdose and/or cases of medication errors correspond to the known side effects of ambroxol hydrochloride at recommended doses and require symptomatic treatment.
Side effects
Skin rash, urticaria, angioedema, itching, anaphylactic reactions (including anaphylactic shock), other hypersensitivity reactions, dysgeusia (taste disorder), nausea, decreased sensitivity in the oral cavity, vomiting, diarrhea, dyspepsia, abdominal pain, dry mouth, drooling, dry throat, decreased sensitivity in the pharynx, shortness of breath (as a symptom of a hypersensitivity reaction).
Interaction
Simultaneous use of the drug and cough suppressants may lead to excessive mucus accumulation due to suppression of the cough reflex, therefore such a combination is possible only after a doctor carefully assesses the ratio of the expected benefit and the possible risk of use.
Storage conditions
Keep out of reach of children.
The medicine does not require special storage conditions.
Shelf life – 5 years.
Be the first to review “Ambroxol Teva tablets 30 mg 20 pcs.” Cancel reply
You may also like

Rinazoline nasal spray 0.5 mg/ml bottle of 10 ml
Solpadeine Active tablets effervescent strip 12 pcs
Rated 4.00 out of 5






Reviews
There are no reviews yet.