No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
Vipratox liniment tube 40 g
$20.72

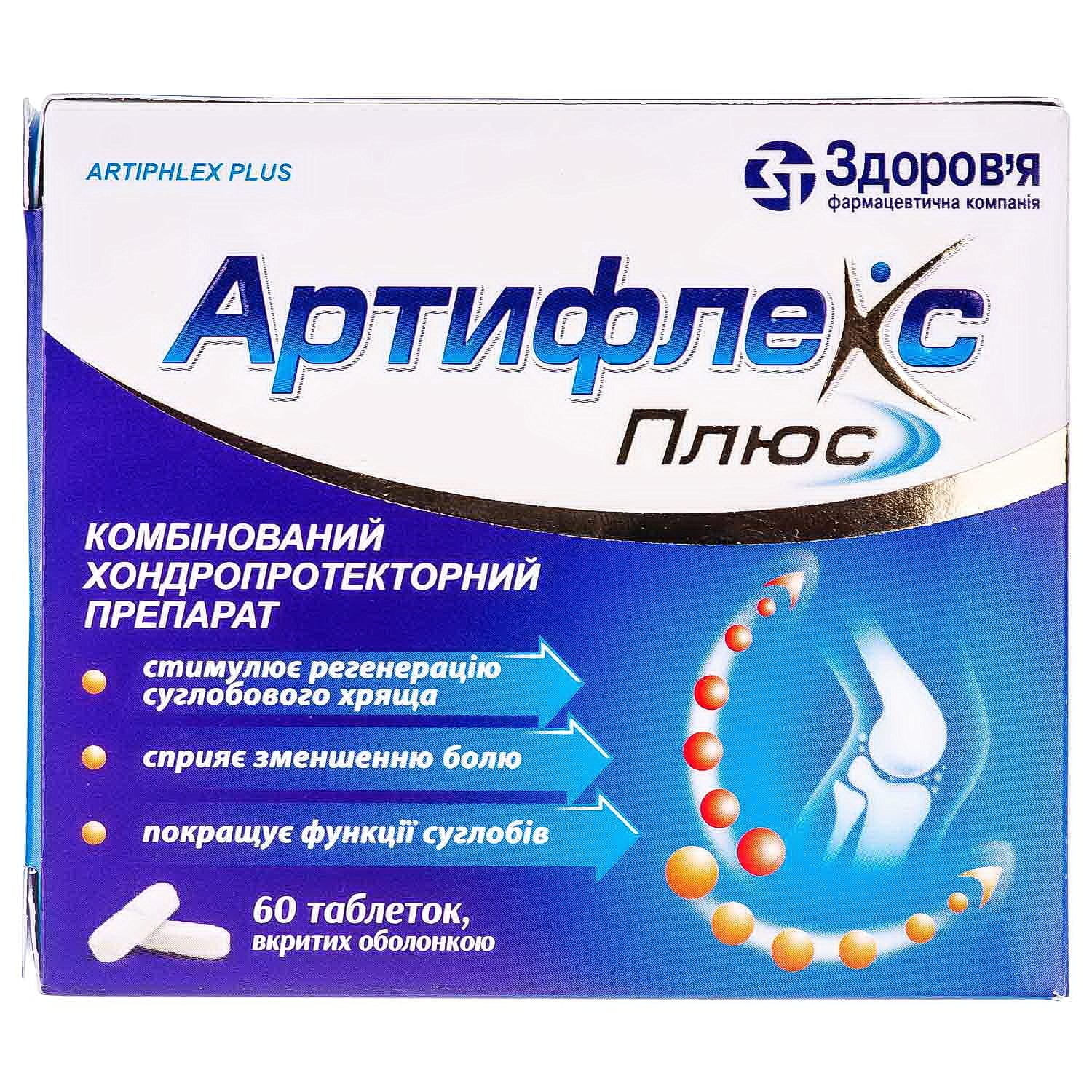
Artiflex Plus film-coated tablets 1000 mg 60 pcs.
$33.44
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, South Korea, Switzerland, United Kingdom, United States and more
In stock
Artiflex Plus 1000 mg – chondroprotective tablets with glucosamine & chondroitin for joint health, cartilage regeneration and pain relief.
Category: Gastrointestinal tract and liver
Brand: Zdorovye
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Composition of the medicinal product:
active ingredients: 1 tablet contains chondroitin sodium sulfate 500 mg, glucosamine hydrochloride 500 mg;
excipients: crospovidone, microcrystalline cellulose, croscarmellose sodium, magnesium stearate, calcium hydrogen phosphate, colloidal anhydrous silicon dioxide, dry mixture “Opadry II white”, containing: titanium dioxide (E 171), talc, polyethylene glycol (macrogol), polyvinyl alcohol.
Dosage form.
The tablets are coated with a film. The tablets are coated with a film of white or almost white color, elongated in shape, with a biconvex surface, with a score.
Pharmacotherapeutic group.
Nonsteroidal anti-inflammatory and antirheumatic drugs. Glucosamine and chondroitin sulfate. ATC code M01A X.
Combined chondroprotective drug that stimulates the regeneration of cartilage tissue. Glucosamine and chondroitin sulfate sodium participate in the biosynthesis of connective tissue, prevent the processes of cartilage destruction and stimulate tissue regeneration.
Chondroitin sulfate is a high-molecular mucopolysaccharide that participates in the construction of the main substance of cartilage and bone tissue. Improves phosphorus-calcium metabolism in cartilage tissue, inhibits enzymes that disrupt the structure and functions of joint cartilage, inhibits the processes of cartilage degeneration. Stimulates the synthesis of glucosaminoglycans, normalizes the metabolism of hyaline tissue, promotes the regeneration of the surface of cartilage and the joint capsule. Has analgesic properties, reduces joint pain, pain at rest and when walking, and manifestations of inflammation. Glucosamine hydrochloride has chondroprotective properties, reduces the deficiency of glycosaminoglycans in the body, participates in the biosynthesis of proteoglycans and hyaluronic acid. Due to its tropism for cartilage tissue, glucosamine hydrochloride initiates the process of sulfur fixation during the synthesis of chondroitin sulfate acid. Glucosamine hydrochloride selectively acts on articular cartilage, is a specific substrate and stimulator of the synthesis of hyaluronic acid and proteoglycans, inhibits the formation of superoxide radicals and enzymes that cause damage to cartilage tissue (collagenase and phospholipase), prevents the destructive effect of glucocorticoids on chondrocytes and disruption of glycosaminoglycan biosynthesis caused by nonsteroidal anti-inflammatory drugs.
Indications for use.
Degenerative-dystrophic diseases of peripheral joints and spine (osteoarthrosis, osteochondrosis, spondyloarthrosis); osteopathies and chondropathy, chondromalacia, periodontopathy; prevention and treatment of joint damage due to physical overload (including sports injuries); recovery period after bone fractures (to accelerate the formation of bone callus), injuries, and operations on the musculoskeletal system.
Contraindication.
Hypersensitivity to any component of the drug. Renal failure in the stage of decompensation, phenylketonuria.
Special precautions.
Use during pregnancy or breastfeeding.
The drug is contraindicated during pregnancy. Breastfeeding should be discontinued during treatment.
Ability to influence the reaction rate when driving or operating other mechanisms.
Unknown.
Children.
The drug is not used in children.
Method of administration and doses.
Take orally, washed down with water.
Adults are prescribed 1-3 tablets per day. It is recommended to start treatment with a higher dose (3 tablets), gradually switching to a maintenance dose. The maintenance dose is determined by the doctor individually. The minimum duration of the course of treatment is 6 weeks; the clinical effect is observed after 2-3 months of using the drug. The clinical effect occurs slowly and persists for a long time after discontinuation of the drug.
Overdose.
In case of accidental overdose, vomiting should be induced. Treatment is symptomatic.
Side effects.
Moderate manifestations of allergic reactions (skin rashes, itching, urticaria, etc.), gastrointestinal disorders (nausea, abdominal pain, flatulence) are possible, which disappear after stopping the drug.
Interaction with other drugs and other types of interactions.
When taken simultaneously, glucosamine enhances the absorption of tetracycline antibiotics in the digestive tract and reduces – penicillin and chloramphenicol. When used simultaneously with glucocorticosteroids and non-steroidal anti-inflammatory drugs, glucosamine and chondroitin may reduce the need for them, as well as for painkillers.
Expiration date.
3 years.
Storage conditions.
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of the reach of children.
Be the first to review “Artiflex Plus film-coated tablets 1000 mg 60 pcs.” Cancel reply
You may also like








Reviews
There are no reviews yet.