No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more

Activated charcoal tablets of ...
$19.19 Original price was: $19.19.$17.39Current price is: $17.39.
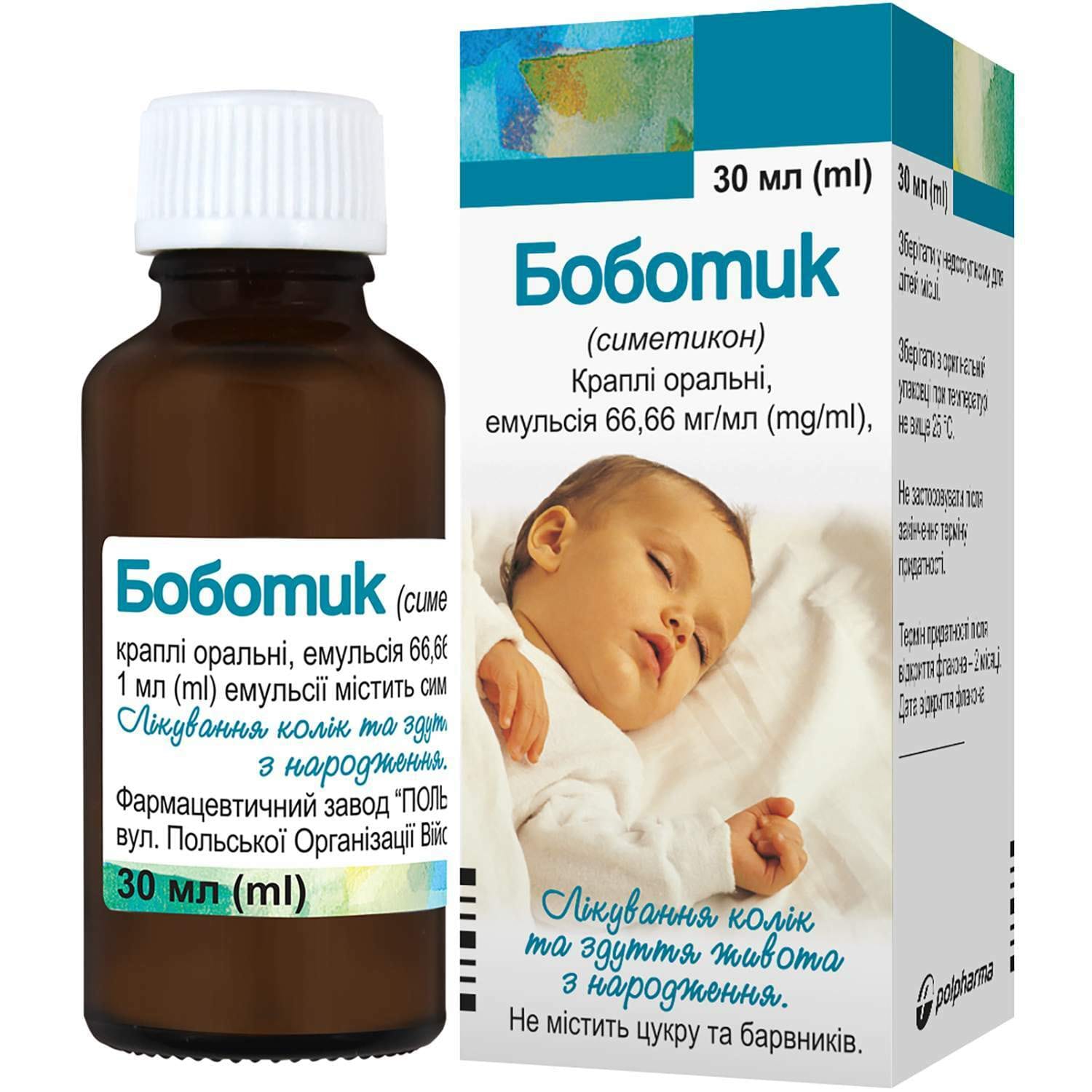
Bobotic oral drops emulsion in a bottle of 30 ml
$23.23
Categories: Gastrointestinal tract and liver, Medicines for children
Brand: Polpharma
Bobotic oral drops with simethicone relieve gas, colic and bloating in children and adults. Suitable for diagnostics prep. Sugar-free formula.
— or —
Composition and form of release
Composition
active ingredient: simethicone (dimethicone activated with silicon dioxide in the form of a 30% emulsion);
1 ml of emulsion contains simethicone (dimethicone activated with silicon dioxide in the form of a 30% emulsion) 66.66 mg; 1 ml contains approximately 27 drops;
excipients: saccharin sodium, carmellose sodium, methyl parahydroxybenzoate (E 218), propyl parahydroxybenzoate (E 216), citric acid monohydrate, raspberry flavoring (ethanol 96%, isopropyl alcohol, flavorings), purified water.
Release form
Oral drops, emulsion.
Pharmacological properties
Simethicone (activated dimethicone) is a combination of methylated linear siloxane polymers stabilized by trimethylsiloxy groups with silicon dioxide. By reducing the surface tension at the interface, it hinders the formation and promotes the destruction of gas bubbles in the nutrient suspension and mucus of the gastrointestinal tract. The gases released in this case can be absorbed by the intestinal walls or excreted due to peristalsis. This prevents the formation of large gas-mucus conglomerates that cause painful bloating. During ultrasound and radiography, it prevents the appearance of image defects, promotes better irrigation of the colon mucosa with contrast agents, preventing the rupture of the contrast film.
Simethicone after oral administration is not absorbed in the gastrointestinal tract and is excreted unchanged in the feces. Due to chemical inertness, it does not affect microorganisms and enzymes present in the gastrointestinal tract. It does not reduce the absorption of food, does not change the reaction and volume of gastric juice.
Indication
Symptomatic treatment of digestive tract disorders associated with gas accumulation, for example, with flatulence, with colic in children; preparation for diagnostic examinations of the abdominal cavity and pelvic organs (X-ray examinations and gastrofibroscopy); as an antifoam in acute poisoning with detergents.
Contraindication
Hypersensitivity to simethicone and/or other components of the drug; intestinal obstruction; obstructive diseases of the gastrointestinal tract.
Application
The drug is taken orally after consulting a doctor.
Shake before use until a homogeneous emulsion is obtained.
To accurately measure the dose of the drug, the bottle should be held vertically during instillation.
For digestive tract disorders caused by gas accumulation
The drug is usually used after meals 3-5 times a day (including before bedtime):
- for children aged from 28 days of life to 1 year, add 16 drops to a bottle of baby food with each feeding or with the help of a small spoon, add before or after breastfeeding;
- children aged 1-6 years – 16 drops (40 mg of simethicone) 3-5 times a day;
- children aged 6-14 years – 16-32 drops 3-5 times a day;
- children over 14 years of age and adults – 32 drops 3-5 times a day.
For convenient use of the drug by young children, it can be mixed in advance with a small amount of boiled, cooled water, baby food, or non-carbonated liquid.
The duration of treatment depends on the presence of complaints and is determined by the doctor individually.
Preparation for diagnostic procedures
One day before the examination, 32 drops are prescribed 3 times a day and 32 drops once in the morning before the examination. In addition to the contrast agent suspension, adults are given 64-128 drops of Bobotic (3-6 ml) per 1 liter of contrast mixture to obtain a double-contrast X-ray image.
As an antidote for surfactant poisoning.
In case of poisoning with detergents, 1.5-6 ml (40-160 drops) of the drug is used in children, and 6-12 ml of the drug is used in adults.
Special instructions
Bobotic does not contain sugar, so it can be used by diabetics. It is not recommended to drink carbonated drinks during the period of Bobotic use. Taking the drug may distort the results of some diagnostic tests, for example, a test using guaiac resin. If complaints recur or persist, you should consult a doctor.
Use during pregnancy and breastfeeding. There is no evidence that simethicone has a teratogenic or embryotoxic effect. The drug can be used during pregnancy and breastfeeding as prescribed by a doctor.
Impact on the ability to drive vehicles or operate other mechanisms. No effect.
Overdose
The drug is chemically inert and is not absorbed in the gastrointestinal tract. There is no information about an overdose of Bobotic. In case of unusual reactions, you should consult a doctor regarding further use of the drug.
Side effects
When taking the drug in recommended doses, no side effects were observed. Allergic reactions (skin rash, itching) are possible.
Interactions
Absorption of oral anticoagulants may be impaired.
Storage conditions
At a temperature not exceeding 25 °C. Store out of the reach of children.
Be the first to review “Bobotic oral drops emulsion in a bottle of 30 ml” Cancel reply
You may also like








Reviews
There are no reviews yet.