No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
Corvalol Darnytsia drops, oral...
$15.09 Original price was: $15.09.$13.69Current price is: $13.69.


Valeriana forte film-coated ta...
$16.39 Original price was: $16.39.$14.89Current price is: $14.89.
[category_image]
Corvaltab Extra film-coated tablets 20 pcs
$17.49 Original price was: $17.49.$15.89Current price is: $15.89.
Corvaltab Extra tablets with valerian and lemon balm help relieve anxiety, stress, and tension naturally. Convenient pack of 20 tablets.
Categories: Nervous system
Brand: Acino
Composition and form of release
Composition
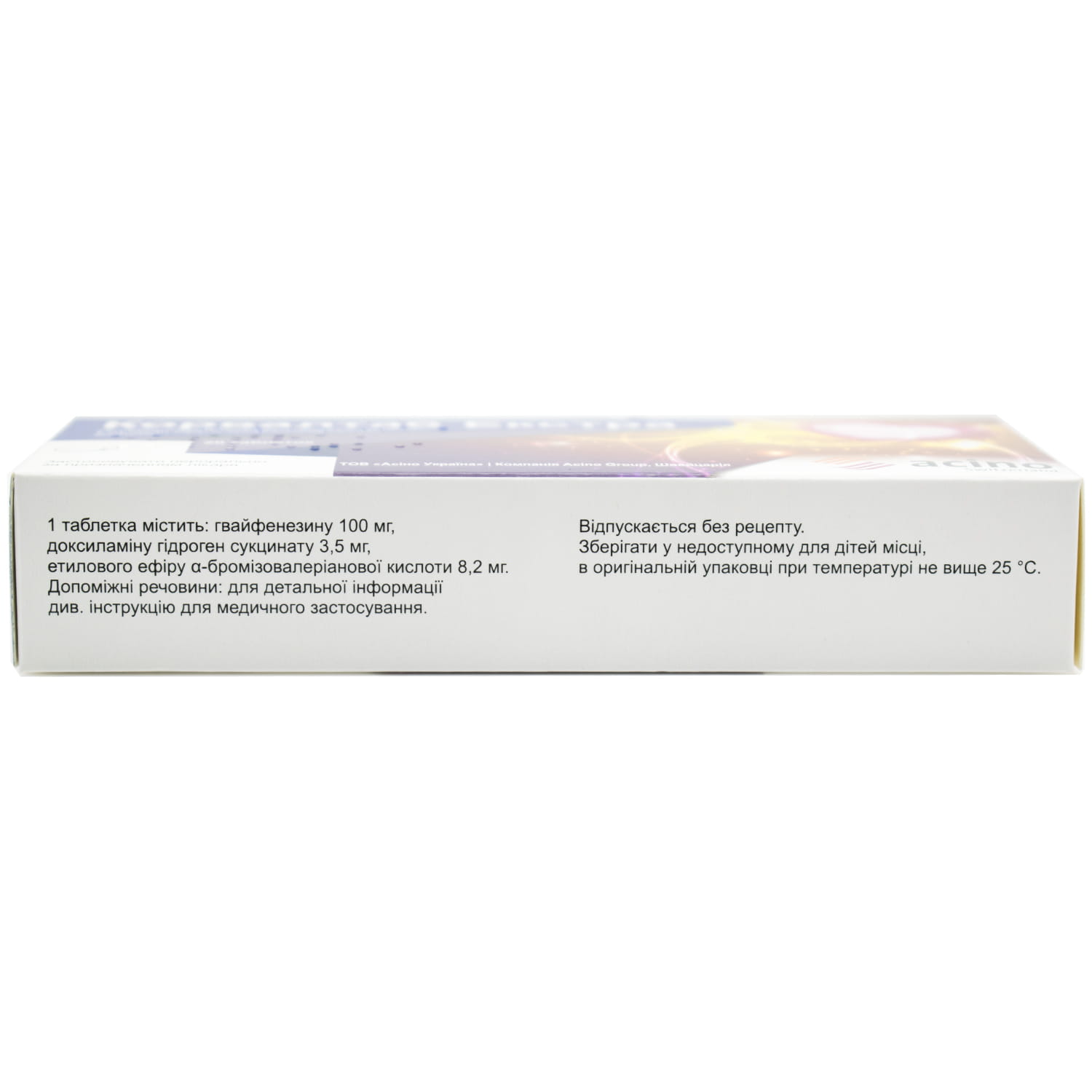
active substances: guaifenesin, doxylamine hydrogen succinate, ethyl ether of α-bromiso-valeric acid;
1 tablet contains guaifenesin 100 mg, doxylamine hydrogen succinate 3.5 mg, ethyl ether of α-bromosovaleric acid 8.2 mg;
excipients: peppermint oil, β-cyclodextrin, maltodextrin, copovidone, crospovidone, silicon dioxide (colloidal hydrophobic), silicon dioxide colloidal aqueous, magnesium stearate; mixture for film coating Opadry II White: polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide (E 171).
Release form
Film-coated tablets.
Pharmacological properties
Pharmacodynamics. vasodilator (coronarolytic), sedative, hypnotic. has a mild antianginal effect. contribute to reducing the excitation of the central nervous system, has a calming effect and facilitates the onset of natural sleep.
Ethyl ether of α-bromosovaleric acid, which is included in the composition of the drug, has antispasmodic, coronaryolytic and sedative effects; in high doses it also causes a mild hypnotic effect.
Guaifenesin causes anxiolytic (anti-anxiety) effect.
Doxylamine hydrogen succinate is a blocker of H1 receptors and exhibits sedative, hypnogenic and antiallergic effects.
Pharmacokinetics. Guaifenesin is quickly (after 30 minutes) absorbed in the gastrointestinal tract. It mainly penetrates into tissues containing acidic mucopolysaccharides. After ingestion, C max is reached in 1-2 hours, and the therapeutic concentration is maintained for 6 hours. T ½ of guaifenesin is about 1 hour. Excreted with sputum and excreted by the kidneys in the form of metabolites, as well as in unchanged form. With doxylamine max, hydrogen succinate in the blood plasma is reached on average 2 hours (T max) after taking the drug.
T ½ of doxylamine from blood plasma is on average 10 hours.
Doxylamine hydrogen succinate is partially metabolized in the liver by demethylation and N-acetylation. T ½ can be significantly increased in the elderly and in patients with renal or hepatic insufficiency. The various metabolites formed during the breakdown of the molecule are not quantitatively significant, since 60% of the dose is found in the urine in the form of unchanged doxylamine.
Indication
Severe spasms of coronary vessels; neurocirculatory dystonia – in complex therapy; neurosis with increased irritability; increased excitability; a mild form of insomnia; dermatosis accompanied by itching.
Contraindication
Hypersensitivity to the components of the drug or antihistamines. severe disorders of kidney and liver function, hepatic porphyria. glaucoma in the patient’s history or family history. urethroprostatic disorders with the risk of urinary retention. severe heart failure. depression and other disorders accompanied by depression of the central nervous system.
Application
The dosage and duration of the treatment course is determined by the doctor individually for each patient. adults are usually prescribed 1 tablet of the drug 2-3 times a day before meals. with a mild form of insomnia, 1-2 tablets are prescribed 30 minutes before bedtime.
Special instructions
You should avoid drinking alcohol while taking the drug.
It should be used with caution in elderly patients due to the risk of dizziness.
During treatment, the syndrome of night apnea (increase in the number and duration of breathing stops) may worsen.
In some cases, a change in the color of urine is possible.
The drug should be used with caution in the following categories of patients with:
- chronic or continuous cough caused by asthma, smoking, chronic bronchitis and emphysema;
- impaired kidney function;
- myasthenia gravis;
- acute gastrointestinal disorders.
Use during pregnancy and breastfeeding
The drug is not prescribed during pregnancy and breastfeeding.
Children
There is no experience of use in children, so the drug is not used in pediatric practice.
To the drivers
During the treatment period, you should refrain from driving or working with other mechanisms that require a quick reaction.
Overdose
Drowsiness, weakness, dizziness, vertigo, gastrointestinal disorders and signs of anticholinergic effects are possible: disturbances, pupil dilation, accommodation paralysis, dry mouth, redness of the face and neck, hyperthermia, sinus tachycardia. the use of the drug in very high doses can cause such symptoms as disturbances, confusion and respiratory depression.
Treatment: discontinuation of the drug, gastric lavage and symptomatic therapy. There is no specific antidote.
Side effects
In some cases, the following side effects may occur:
from the side of the nervous system: drowsiness, light dizziness;
from the digestive tract: nausea, vomiting;
from the side of the immune system: allergic reactions (including skin rashes, itching, urticaria).
These phenomena are eliminated by reducing the dose or stopping the drug.
With long-term use of the drug in high doses, the development of bromism is possible.
Diarrhea, constipation, dry mouth, impaired accommodation, pronounced palpitations.
Daytime drowsiness: when such an effect develops, it is necessary to reduce the dose.
There are isolated reports of the formation of calculi in the urinary bladder or in the kidneys in patients who have taken high doses of guaifenesin for a long time.
In case of unwanted side reactions, consult a doctor.
Interactions
With simultaneous use with other CNS-depressant drugs, mutual enhancement of the effect is possible, as well as an increase in the effect of ethanol. the effect of the drug increases against the background of alcohol consumption.
It should be taken into account that in the case of using a combination of the drug with:
- atropine and atropine-like drugs (imipramine antidepressants, anticholinergic antiparkinsonian drugs, atropine antispasmodic drugs, disopyramide, phenothiazine neuroleptics) may cause such side effects as urinary retention, constipation, dry mouth;
- antidepressants, morphine derivatives (pain relievers and cough relievers), neuroleptics; barbiturates, benzodiazepines, antihypertensive agents of central action may increase CNS depression.
Storage conditions
In the original packaging at a temperature not higher than 25 °C.
ACIN-PIM-122016-005
Be the first to review “Corvaltab Extra film-coated tablets 20 pcs” Cancel reply
You may also like





Reviews
There are no reviews yet.