No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
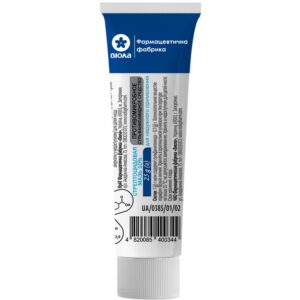
Exik cream 1% tube 15 g
$20.38
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, South Korea, Switzerland, United Kingdom, United States and more
In stock
Exik treats fungal infections of the skin and nails, providing strong antifungal, antibacterial and anti-inflammatory action with low systemic absorption.
Categories: Dermatology
Brand: Vishpha
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Pharmacological properties
Pharmacodynamics. Exik is an antifungal agent of the allylamine class. The active component of the drug is naftifine hydrochloride. The mechanism of action of naftifine hydrochloride is inhibition of the action of ergosterol.
Naftifine is active against dermatophytes such as Trichophyton, Epidermophyton and Microsporum, yeasts (Candida), molds (Aspergillus) and other fungi (e.g. Sporothrix schenckii). Naftifine has fungicidal action against dermatophytes and Ampergillus, and against yeasts it has fungicidal or fungistatic action depending on the strain of the microorganism.
Also, the drug Exik is characterized by antibacterial activity against gram-positive and gram-negative microorganisms that can cause secondary bacterial infections along with mycotic lesions.
In addition, the drug has powerful anti-inflammatory properties.
Pharmacokinetics. Naftifine hydrochloride is rapidly absorbed and forms stable antifungal concentrations in different layers of the skin. About 4% of the dose applied to the skin is absorbed, so the systemic exposure of the active substance is extremely low. Only trace amounts of naftifine are found in blood plasma and urine. The active substance is almost completely metabolized; metabolites do not have antifungal activity and are excreted in feces and urine. T ½ – 2-4 days.
Indication
Local treatment of fungal infections caused by pathogens sensitive to naftifine:
- fungal infections of the skin and skin folds;
- interdigital mycoses;
- fungal nail infections;
- tinea versicolor;
- inflammatory dermatomycoses, accompanied by itching or without it.
Application
Exik cream or solution should be applied to the affected skin surface and adjacent areas 1 g/day after thorough cleansing and drying, capturing approximately 1 cm of healthy skin from the edge of the affected area.
The duration of therapy for dermatomycoses is 2-4 weeks (if necessary – up to 8 weeks), for candidiasis – 4 weeks, for infectious nail lesions – up to 6 months.
In case of mycotic nail lesions, the drug is recommended to be used 2 times a day. Before the first use, the affected part of the nail should be removed as much as possible with scissors or a nail file (to facilitate this procedure, on the recommendation of a doctor, the nails can be treated with a special softening agent).
In order to prevent relapses, therapy should be continued for at least 2 weeks after the disappearance of the main symptoms of the disease.
Children. Data on the efficacy and safety of the drug in children are insufficient, therefore it is not recommended to prescribe Exik to patients in this age category.
Contraindication
Hypersensitivity to naftifine or other components of the drug. The drug should not be applied to the wound surface. Do not use for the treatment of eye diseases.
Side effects
The frequency of adverse reactions is defined according to the medical dictionary for regulatory activities meddra as follows: very common (≥1/10), common (≥1/100 to 1/10), uncommon (≥1/1000 to 1/100), rare (≥1/10,000 to 1/1000), very rare (1/10,000), frequency unknown (cannot be estimated from the available data).
General disorders: frequency unknown – local reactions may rarely occur: dry skin, redness and burning sensation, erythema, itching, local irritation.
Special instructions
The drug should be used externally only for nail and skin diseases!
The cream contains cetyl alcohol and stearyl alcohol, which may cause local skin reactions (e.g. contact dermatitis).
The topical solution contains ethanol, so avoid getting the solution in the eyes and on open wounds.
The solution contains propylene glycol, which may cause skin irritation.
Use during pregnancy or breastfeeding. Data on the use of naftifine in pregnant women are absent or limited. The results of animal studies do not indicate direct or indirect harmful effects of the drug on reproductive function. The drug should be used during pregnancy or breastfeeding only if absolutely necessary after a careful assessment of the benefit/risk ratio, which is determined by the doctor.
Breastfeeding mothers should avoid getting the cream on the baby’s skin and digestive tract.
The ability to influence the reaction rate when driving or working with other mechanisms. Does not affect.
Interactions
Drug interaction studies have not been conducted.
Overdose
Acute overdose with topical application of naftifine has not been observed.
Systemic intoxication with external use of the drug is unlikely due to the fact that a small amount of the active substance is absorbed through the skin.
In case of accidental ingestion of the drug, symptomatic treatment should be initiated.
Storage conditions
Cream: at a temperature not exceeding 30 °C. Do not freeze.
Cutaneous solution: at a temperature not exceeding 30 °C. After opening the bottle, the solution should be stored for 6 months at a temperature not exceeding 25 °C.
Keep out of reach of children.
You may also like