No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
Helpex Anticold DX tablets, 100 pcs
$63.45
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, South Korea, Switzerland, United Kingdom, United States and more
In stock
Helpex Anticold DX tablets provide fast relief from cold and flu symptoms, including cough, fever, nasal congestion, and pain. Convenient daily use.
Categories: Cold and flu
Brand: MoviHealth
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Indications
Helpex Anticold DX tablets are used for the symptomatic treatment of acute respiratory viral infections and influenza: relief of dry irritating cough, reduction of elevated body temperature, alleviation of nasal congestion, reduction of nasal mucosal swelling, relief of body aches and headache.
Composition
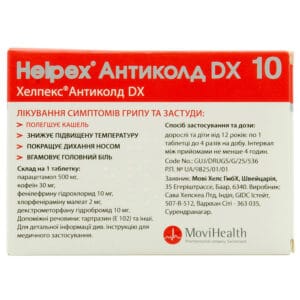
One tablet contains (active substances):
- Paracetamol — 500 mg
- Caffeine — 30 mg
- Phenylephrine hydrochloride — 10 mg
- Chlorpheniramine maleate — 2 mg
- Dextromethorphan hydrobromide — 10 mg
Excipients: corn starch, microcrystalline cellulose, povidone, tartrazine (E102), magnesium stearate, talc, sodium starch glycolate (type A), brilliant blue (E133).
Contraindications
- Hypersensitivity to any component of the product or to other xanthine derivatives (theophylline, theobromine)
- Decompensated heart failure, ventricular tachycardia, acute myocardial infarction, cardiac conduction disorders, severe ischemic heart disease, severe arterial hypertension, pronounced atherosclerosis, peripheral arterial thrombosis
- Pheochromocytoma
- Bronchial asthma, emphysema, chronic obstructive pulmonary disease (risk of respiratory failure)
- Stenosing peptic ulcer of the stomach or duodenum, pyloroduodenal obstruction
- Severe hepatic or renal impairment, acute pancreatitis, hepatitis
- Prostatic adenoma with urinary retention, bladder neck obstruction
- Blood disorders (including severe anemia, leukopenia)
- Glucose-6-phosphate dehydrogenase deficiency, congenital hyperbilirubinemia
- Epilepsy, increased excitability, sleep disorders
- Hyperthyroidism, diabetes mellitus, alcoholism
- Elderly patients (over 60 years)
- Glaucoma
- Concurrent use with tricyclic antidepressants, β-blockers, SSRIs, MAO inhibitors (and within 2 weeks after MAOI discontinuation)
Dosage and Administration
Adults and children over 12 years: 1 tablet up to 4 times daily. Maintain at least a 4-hour interval between doses. Treatment duration should not exceed 5 days. Maximum duration without medical advice — 3 days. Take 1 hour after food with plenty of water.
Do not exceed the recommended dose.
Special Precautions
Pregnancy and breastfeeding: Not to be used during pregnancy and lactation.
Children: For treatment of children from 12 years of age only.
Drivers and machine operators: Avoid driving, operating machinery, and performing hazardous activities while taking the medicine.
Overdose
Patients with risk factors may develop symptoms of paracetamol overdose when combined with other drugs or conditions that increase oxidative stress and deplete hepatic glutathione (prolonged fasting, sepsis, diabetes).
Early (first 24 hours) symptoms: pallor, nausea, vomiting, anorexia, abdominal pain. Other possible signs: CNS excitation or depression, sweating, dizziness, sleep disturbances, arrhythmias, tachycardia, extrasystoles, tremor, hyperreflexia, seizures, pancreatitis.
Adverse Reactions
Immune system: hypersensitivity reactions including itching, skin and mucosal rashes (generalized erythematous rash, urticaria), anaphylactic shock, angioedema, erythema multiforme (including Stevens–Johnson syndrome), toxic epidermal necrolysis.
Central nervous system: psychomotor agitation, disorientation, anxiety, irritability, sleep disturbances, insomnia, drowsiness, dizziness, confusion, hallucinations, depression, tremor, paresthesia, tinnitus, headache; in rare cases — coma, seizures, dyskinesia, behavioral changes, generalized weakness, increased sweating.
Respiratory system: bronchospasm in patients sensitive to aspirin and other NSAIDs.
Eye disorders: visual disturbances, accommodation disorders, mydriasis, increased intraocular pressure, dry eyes.
Gastrointestinal: nausea, vomiting, heartburn, dry mouth, epigastric discomfort/pain, diarrhea, hypersalivation, decreased appetite, ulcer exacerbation, flatulence, constipation.
Liver: impaired liver function, elevated hepatic enzymes (usually without jaundice), hepatonecrosis with high doses.
Endocrine: hypoglycemia up to hypoglycemic coma.
Hematologic: anemia (including hemolytic), bruising/bleeding; sulfhemoglobinemia and methemoglobinemia (cyanosis, dyspnea, chest pain). Long-term high doses may cause aplastic anemia, pancytopenia, agranulocytosis, neutropenia, leukopenia, thrombocytopenia.
Renal/urinary: nephrotoxicity (including papillary necrosis) with high doses, urinary retention, dysuria, interstitial nephritis, increased creatinine clearance, increased excretion of sodium and calcium, aseptic pyuria, renal colic.
Cardiovascular: arterial hypertension, tachycardia or reflex bradycardia, arrhythmia, dyspnea, chest pain.
Concurrent use with caffeine-containing products may increase caffeine-related adverse effects (dizziness, excitability, insomnia, anxiety, irritability, headache, GI disturbances, palpitations).
Storage Conditions
Store in the original packaging at a temperature not exceeding 25 °C, out of reach of children.
Shelf life: 4 years.
Be the first to review “Helpex Anticold DX tablets, 100 pcs” Cancel reply
You may also like

Nasalong nasal spray dosed 0.05% bottle of 10 g
Solpadeine Active tablets effervescent strip 12 pcs
Rated 4.00 out of 5










Reviews
There are no reviews yet.