No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more

Anaferon children’s tabl...
$20.99 Original price was: $20.99.$19.09Current price is: $19.09.
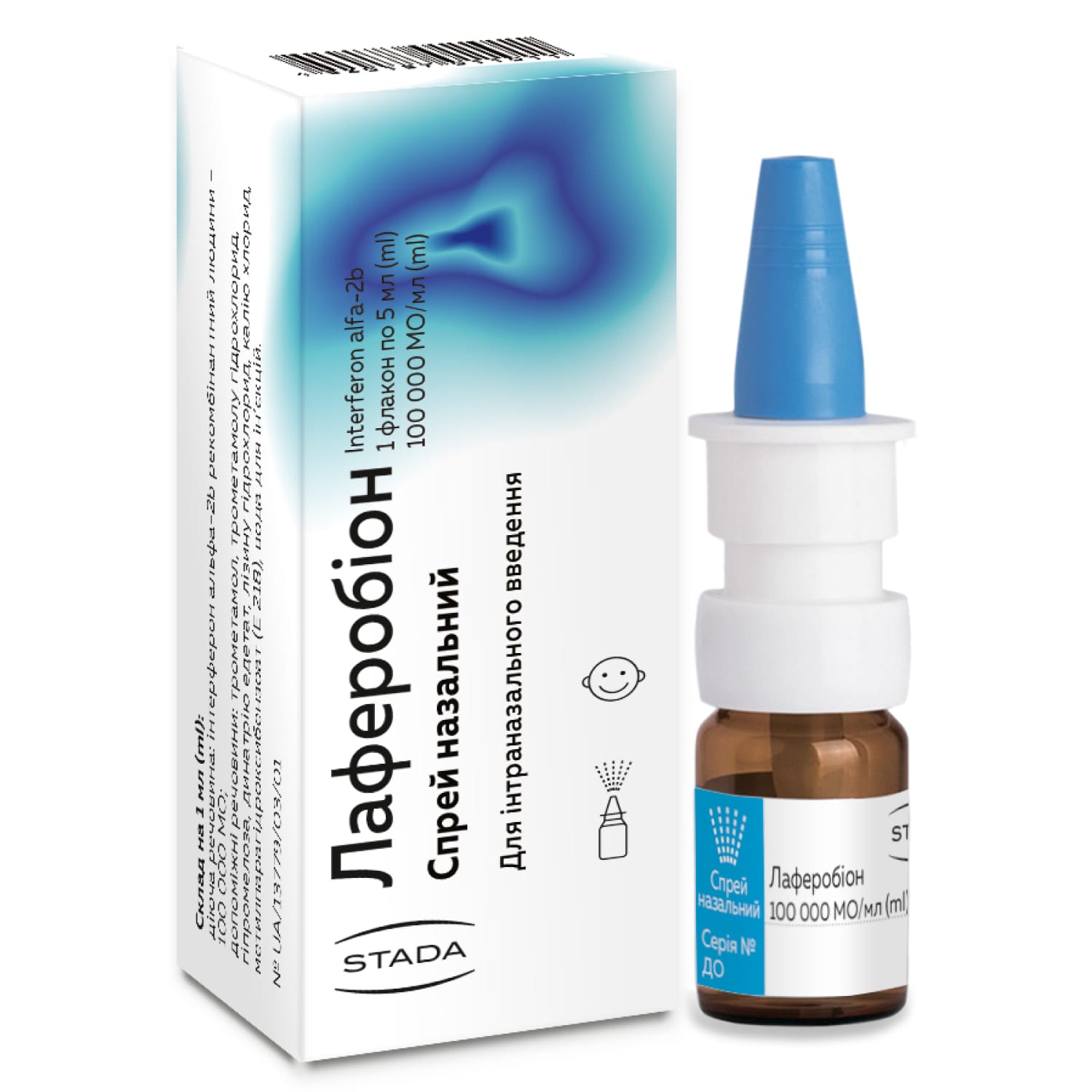
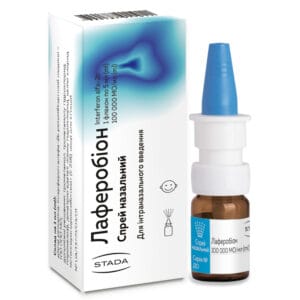
Laferobion nasal spray 100,000 IU/ml bottle sealed with a micro-dropper 5 ml
$18.65
Laferobion nasal spray with interferon alfa-2b for ARVI prevention and treatment. For adults and children 1+. Antiviral immunostimulant.
— or —
Laferobion – antiviral, antimicrobial, anti-inflammatory, immunomodulatory, antiproliferative agent. Immunostimulant. Interferon alpha-2b for the prevention and treatment of acute respiratory viral infections (ARVI):
- in patients who frequently and persistently suffer from upper respiratory tract diseases;
- in contact with patients with acute respiratory viral infections;
- during hypothermia;
- with seasonal increase in morbidity.
Composition of Laferobion nasal spray
- active ingredient: interferon alfa-2b;
- 1 ml of the drug contains 100,000 IU of recombinant human interferon alpha-2b;
- Excipients: trometamol, trometamol hydrochloride, hypromellose, disodium edetate, lysine hydrochloride, potassium chloride, methyl parahydroxybenzoate (E 218), water for injections.
Contraindication
Hypersensitivity to interferon alfa-2b and other components of the drug, severe forms of allergic diseases in history; thyroid dysfunction; the presence of severe visceral disorders in patients with Kaposi’s sarcoma; severe cardiovascular diseases; psoriasis; severe liver and/or kidney dysfunction; epilepsy and other diseases of the central nervous system (including functional); chronic hepatitis against the background of progressive or decompensated cirrhosis of the liver; chronic hepatitis in patients receiving or recently receiving immunosuppressant therapy (except for a short course of corticosteroid therapy); autoimmune hepatitis or other autoimmune diseases in history. Suppression of myeloid hematopoiesis. Pregnancy and breastfeeding.
Method of application
The drug is injected into each nasal passage.
1 spray dose = 1 short press on the dispenser.
Nasal spray application schedule:
- Remove the protective cap from the bottle.
- Activate the micro-doser-sprayer by pressing (test spray).
- While in an upright position, place the tip of the nozzle into the nasal passage and press the pump. Repeat the introduction into the second nasal passage.
- After use, close the bottle with the cap.
At the first signs of SARS (within 5 days)
Adults – 3 spray doses in each nasal passage 5–6 times a day (single dose – 30,000 IU, daily dose – 150,000 – 180,000 IU).
Children. Children aged 1 to 3 years – 2 spray doses in each nasal passage 3-4 times a day (single dose – 20,000 IU, daily dose – 60,000 – 80,000 IU).
Children aged 3 to 14 years – 2 spray doses in each nasal passage 4–5 times a day (single dose – 20,000 IU, daily dose – 80,000 – 100,000 IU).
Children aged 14 to 18 years – 3 spray doses in each nasal passage 5–6 times a day (single dose – 30,000 IU, daily dose – 150,000 – 180,000 IU).
For the prevention of respiratory viral infections in adults
In case of contact with a sick person and hypothermia – 3 spray doses 2 times a day for 5-7 days. If necessary, the preventive courses are repeated. In case of a single contact, a single application is sufficient.
During seasonal increases in incidence – once in the morning with an interval of 1–2 days.
Application features
The drug contains methyl parahydroxybenzoate, which may cause allergic reactions, including delayed ones, and in exceptional cases, bronchospasm.
The drug should not be used if the integrity and labeling of the package is broken, if the physical properties (color or transparency of the liquid) change, and after the expiration date.
To avoid the spread of infection, individual use of the vial is recommended.
Use during pregnancy or breastfeeding
Use is contraindicated.
Children
Use in children over 1 year of age at the first signs of SARS.
Ability to influence reaction speed when driving vehicles or other mechanisms
Does not affect.
Overdose of Laferobion nasal spray
There is no data on overdose of the drug.
Adverse reactions
General disorders: often – dose-dependent flu-like syndrome (chills, fever, headache and muscle pain, joint pain, fatigue, lethargy, sweating); rarely – nausea, vomiting, dizziness, hot flashes. Electrolyte imbalance. Hypersensitivity reactions to the drug, including anaphylactic shock, Quincke’s edema, are possible.
Skin and subcutaneous tissue disorders: skin rash (in isolated cases), including herpetic, itching, hyperemia, skin edema, urticaria, dry skin, alopecia.
On the part of the endocrine system: thyroid dysfunction.
From the organs of vision: visual impairment, conjunctival hyperemia.
On the part of the digestive tract: loss of appetite, increased levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase.
Hepatobiliary system: liver dysfunction.
Storage conditions of Laferobion nasal spray
Store in the original packaging to protect from light at a temperature of 2 to 8 ºС.
Keep out of reach of children.
Shelf life 2 years. Shelf life after opening the bottle, if stored at a temperature of 2 to 8 º C, is 10 days.
Be the first to review “Laferobion nasal spray 100,000 IU/ml bottle sealed with a micro-dropper 5 ml” Cancel reply
You may also like






Reviews
There are no reviews yet.