No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark
🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇸🇰 Slovakia🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of America
and more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark
🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇸🇰 Slovakia🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of America
and more
Microlax rectal solution (micr...
$40.29 Original price was: $40.29.$36.59Current price is: $36.59.


Picolax tablets of 7.5 mg, bli...
$15.69 Original price was: $15.69.$14.29Current price is: $14.29.
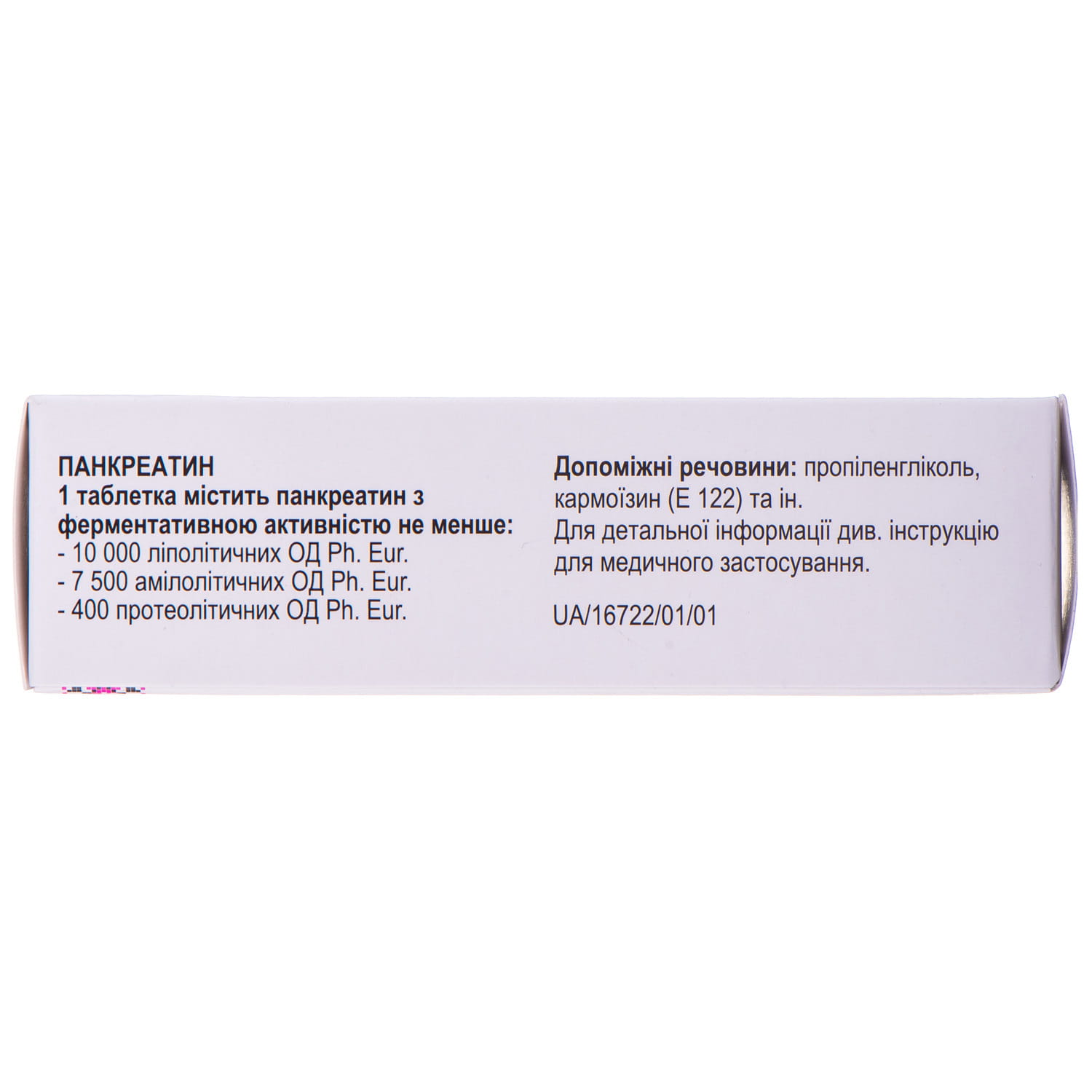
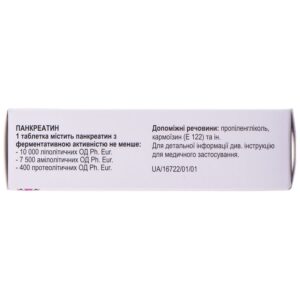
Pancreazim 10000 gastro-resistant tablets 50 pcs
$19.59 Original price was: $19.59.$17.79Current price is: $17.79.
Free Worldwide Shipping
to: Australia, Canada, Czechia, Denmark, Estonia, Ireland, Israel, Italy, Japan, Mexico, Poland, Slovakia, South Korea, Switzerland, United Kingdom, United States and more
In stock
Pancreazim 10000 tablets support digestion with pancreatic enzymes, aiding fat, protein, and carb breakdown for better gastrointestinal health.
Categories: Gastrointestinal tract and liver
Brand: Technolog
Payment
PayPal, Debit or Credit card, Google Pay, Apple Pay
Pancreazym 10000 tablets are used for the following indications
- Diseases that are accompanied by a violation of the process of digesting food due to insufficient secretion of digestive enzymes by the pancreas, such as chronic pancreatitis.
- Conditions after simultaneous resection of the stomach and small intestine, functional acceleration of the passage of food through the intestines, intestinal disorders, simultaneous consumption of hard-to-digest vegetable, fatty and unusual food.
- Intestinal bloating.
- Preparation for x-ray or ultrasound diagnostic studies.
Composition
- active substance: pancreatin;
- 1 gastroresistant tablet contains pancreatin with an enzymatic activity of at least 10,000 lipolytic units of Ph. Eur., 7,500 amylolytic units Ph. Eur., 400 proteolytic units Ph. Eur.;
- auxiliary substances: sodium chloride, colloidal anhydrous silicon dioxide, microcrystalline cellulose, crospovidone, croscarmellose sodium, povidone 25, magnesium stearate, methacrylate copolymer dispersion, talc, propylene glycol, titanium dioxide (E 171), carmoisin (E 122).
Contraindication
Increased sensitivity to the active substance and other components of the medicinal product. Acute pancreatitis, exacerbation of chronic pancreatitis, intestinal obstruction.
Adverse reactions
From the side of the cardiovascular system: the frequency is unknown: tachycardia.
From the side of the immune system: very rarely: allergic reactions of the immediate type (skin rash, itching, sneezing, lacrimation, bronchospasm), anaphylactic reactions. The frequency is unknown: carmoisin dye (E 122) can cause allergic reactions.
From the gastrointestinal tract: very rarely: when using high doses of pancreatin (more than 10,000 units of Ph. Eur. lipase / kg of body weight / day), constrictions may form in the ileocecal area and in the ascending part of the colon; diarrhea, abdominal pain, nausea, vomiting, change in the nature of stools; possible development of intestinal obstruction, constipation.
From the side of the skin and subcutaneous tissues: the frequency is unknown: urticaria, hyperemia, itching, angioedema.
From the genitourinary system: the frequency is unknown: increased excretion of uric acid with urine is possible, especially when using high doses of pancreatin. To avoid the formation of uric acid stones in such patients, the content of uric acid in the urine should be monitored.
General disorders: frequency unknown: feeling of heat, general weakness.
Method of application
The dose of the drug depends on the deficiency of pancreatic enzymes in the duodenum and is set individually.
If there are no other recommendations, as well as in case of consumption of difficult-to-digest vegetable food, fatty or unusual food, take 1-2 tablets. In other cases mentioned above, when digestive disorders occur, the recommended dose is 2–4 tablets. If necessary, the dose of the drug can be increased. Increasing the dose in order to reduce symptoms of the disease, such as steatorrhea or abdominal pain, should be carried out only under the supervision of a doctor. The daily dose of lipase should not exceed 15,000 – 20,000 IU Ph. Eur. per 1 kg of body weight.
Tablets should be taken during meals, swallowing whole and with a large amount of liquid, for example, 1 glass of water.
The duration of treatment depends on the course of the disease and is determined by the doctor individually.
Features of application
Use during pregnancy or breastfeeding
During pregnancy or breastfeeding, the use of the drug should be avoided.
Children
There is no experience of using the drug in children, so it is not recommended to prescribe it to this age category of patients.
The ability to influence the speed of reaction when driving vehicles or other mechanisms
No influence on the ability to drive a car and other mechanical means was observed.
Overdose
Symptoms Side effects may increase. Hyperuricemia and hyperuricosuria have been observed when taking extremely high doses of other pancreatic powder preparations.
Treatment. Drug discontinuation, adequate hydration, and symptomatic therapy.
Interaction with other medicinal products and other types of interactions
When using pancreatin, a decrease in iron absorption is possible, therefore, if necessary, additional iron preparations should be prescribed.
Simultaneous use of antacids that contain calcium carbonate and/or magnesium hydroxide can reduce the effectiveness of pancreatin.
Simultaneous use with tannin, alcohol-containing products can lead to a decrease in the effectiveness of pancreatin.
With simultaneous use of bicarbonates and cimetidine with large doses of pancreatic enzymes, it is recommended to periodically analyze the concentration of folic acid salts in the blood serum and provide additional folic acid intake, if necessary.
Folic acid. Absorption of folic acid may be reduced in patients taking pancreatic enzyme preparations, so monitoring of folic acid levels is recommended during concomitant use.
Acarbose, miglitol. It is possible that pancreatic enzyme preparations may reduce the effect of these antidiabetic agents. Therefore, during concomitant use with pancreatin, it is recommended to monitor the effect of antidiabetic agents on the patient’s blood sugar level.
Storage conditions
Store in the original packaging at a temperature not higher than 25 ºС.
Keep out of the reach of children.
Be the first to review “Pancreazim 10000 gastro-resistant tablets 50 pcs” Cancel reply
You may also like






Reviews
There are no reviews yet.