No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
[category_image]
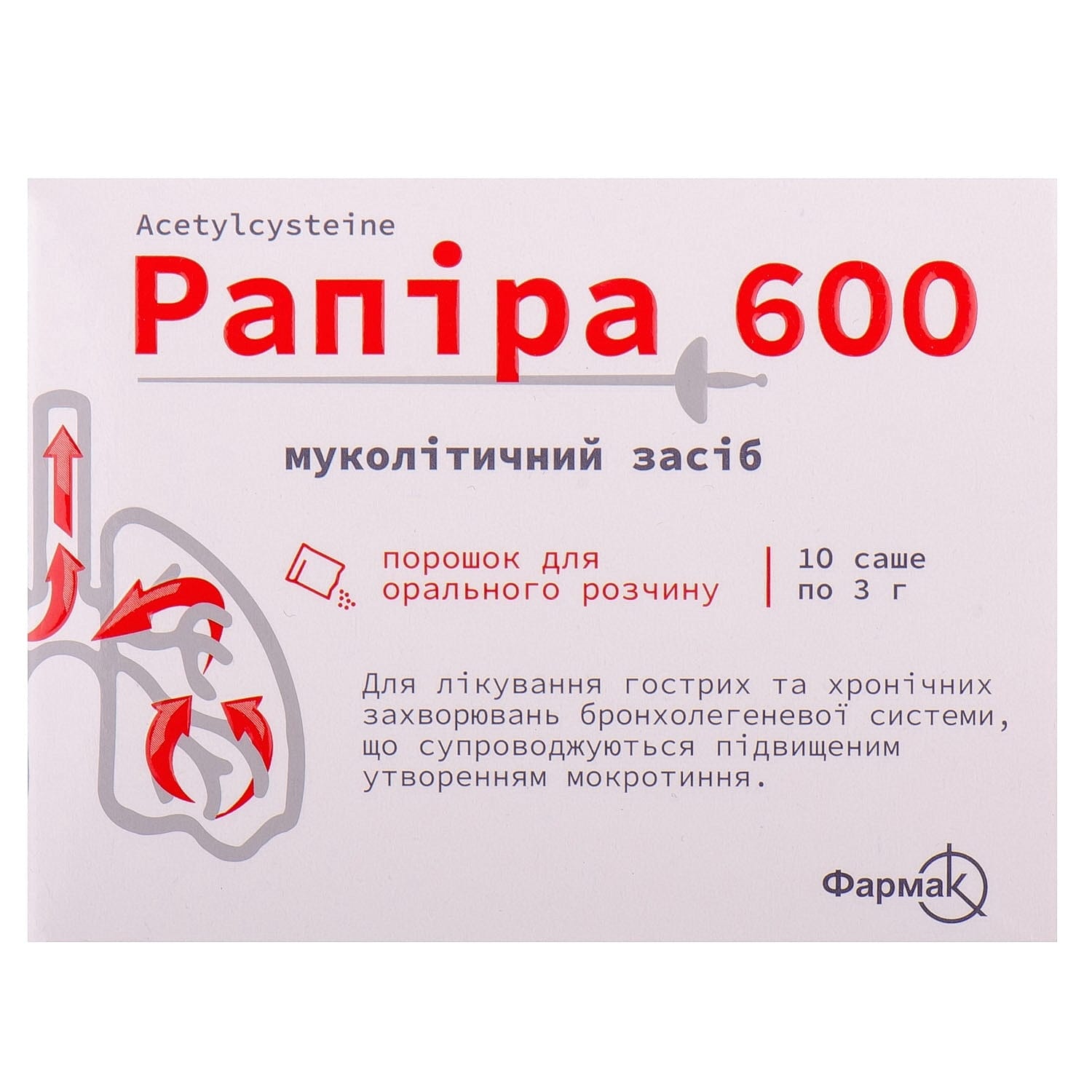
Rapira 600 powder for oral solution 600 mg in a sachet 3 g 10 pcs.
$23.85
Rapira 600 powder with acetylcysteine helps thin mucus and ease cough in acute and chronic lung conditions, supporting effective respiratory relief.
Categories: Cold and flu
Brand: Farmak
Powder for oral solution “Rapira 600” is indicated for use:
- for the treatment of acute and chronic diseases of the bronchopulmonary system, accompanied by increased sputum production;
- in case of paracetamol overdose.
Composition
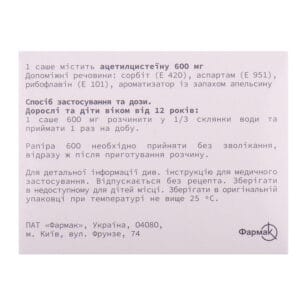
The active substance is acetylcysteine (one sachet contains 600 mg of acetylcysteine).
Excipients: sorbitol (E 420), aspartame (E 951), riboflavin (E 101), orange flavoring.
Contraindication
- known hypersensitivity to acetylcysteine or any of the excipients;
- gastric and duodenal ulcer in the acute stage;
- hemoptysis;
- pulmonary hemorrhage.
Method of application
Adults and children over 12 years of age: dissolve one 600 mg sachet in 1/3 cup of water and take once a day; the duration of the treatment course is determined by the doctor individually depending on the nature of the disease (acute or chronic).
Paracetamol overdose: in the first 10 hours after taking the toxic substance, take this drug as soon as possible at the rate of 140 mg/kg, then at the rate of 70 mg/kg every 4 hours for 1-3 days.
The drug must be taken immediately after preparing the solution.
Application features
Pregnant women
Clinical data on the use of acetylcysteine in pregnant women are limited. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryo-fetal development, parturition or postnatal development.
There is no information on the penetration into breast milk. The drug should be taken during pregnancy and breastfeeding only after a careful assessment of the benefit/risk ratio.
Children
Apply to children aged 12 and over.
Drivers
There is no evidence that acetylcysteine affects the ability to drive and use other mechanisms.
Overdose
There are no reports of overdose with oral acetylcysteine formulations. Volunteers have taken 11.6 g of acetylcysteine per day for three months without any serious adverse effects. Acetylcysteine at a dose of 500 mg/kg/day does not cause overdose.
Symptoms: Overdose may manifest as gastrointestinal symptoms such as nausea, vomiting and diarrhea.
Treatment: There is no specific antidote for acetylcysteine poisoning, therapy is symptomatic.
Side effects
On the part of the immune system: infrequently – hypersensitivity; very rarely – anaphylactic shock, anaphylactic / anaphylactoid reactions.
Blood and lymphatic system disorders: not known – anemia.
From the nervous system: infrequently – headache.
From the side of the organs of hearing and labyrinth: infrequently – tinnitus.
From the respiratory system: unknown – rhinorrhea.
Cardiac: infrequently – tachycardia.
Vascular disorders: very rarely – hemorrhages.
From the side of the chest and mediastinum: rarely – bronchospasm, dyspnea.
From the gastrointestinal tract: infrequently – vomiting, diarrhea, stomatitis, abdominal pain, nausea; rarely – dyspepsia; unknown – unpleasant smell from the mouth.
Skin and subcutaneous tissue disorders: infrequently – urticaria, rash, angioedema, itching; unknown – eczema.
General disorders and administration site conditions: uncommon – hyperthermia; unknown – facial edema.
Investigations: uncommon – decreased blood pressure.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C, out of the reach of children.
Shelf life – 2 years.
Be the first to review “Rapira 600 powder for oral solution 600 mg in a sachet 3 g 10 pcs.” Cancel reply
You may also like









Reviews
There are no reviews yet.