No products in the cart.
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
We deliver to:
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
[category_image]
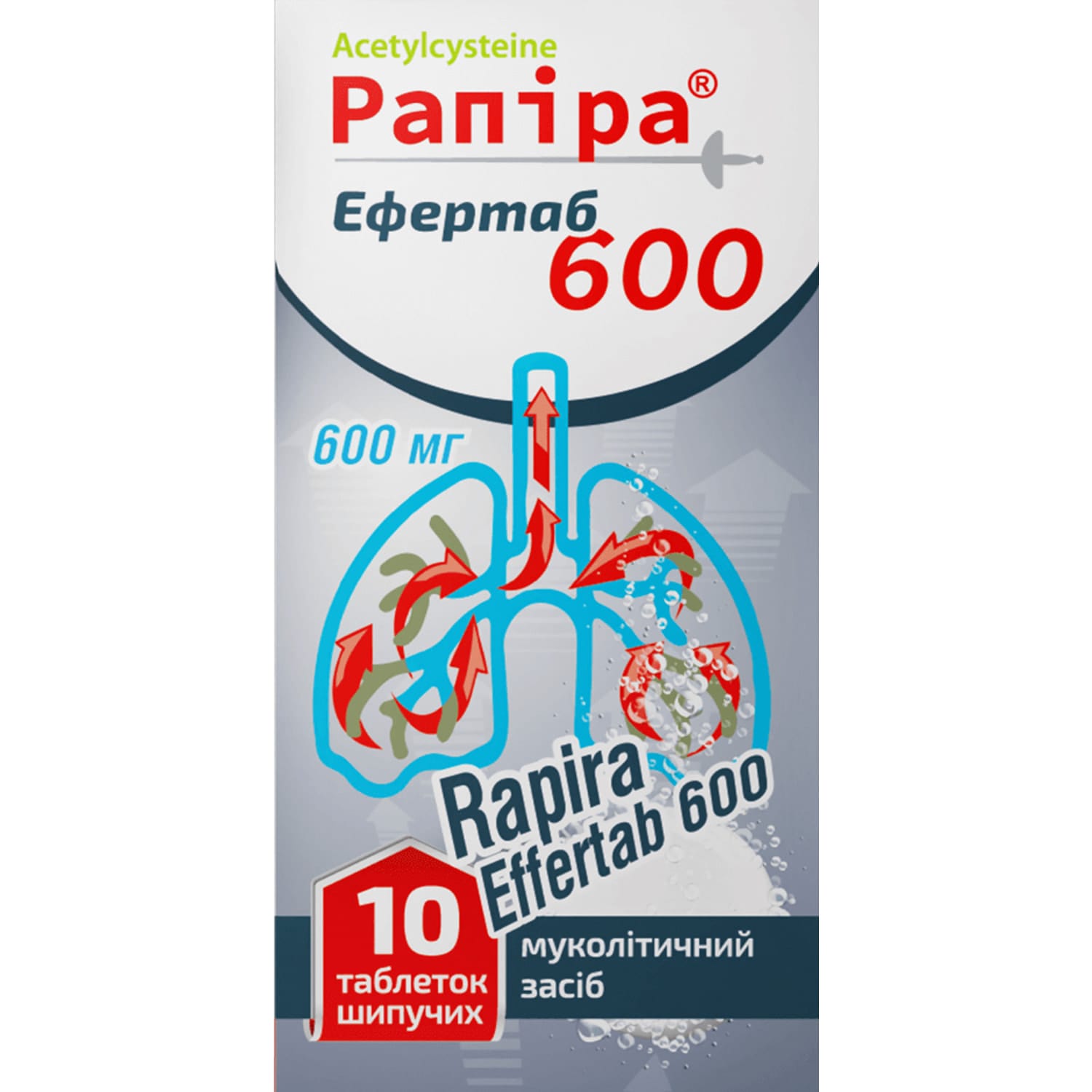
Rapira Efertab 600 effervescent tablets 600 mg tube 10 pcs.
$22.94
Rapira Efertab 600 with acetylcysteine helps thin mucus and ease coughing in acute and chronic lung conditions, and is also used in paracetamol overdose.
Categories: Cold and flu
Brand: Farmak
Rapira Efertab 600 is a mucolytic agent for the treatment of acute and chronic diseases of the bronchopulmonary system, accompanied by increased sputum production.
Paracetamol overdose.
Composition
- active ingredient: acetylcysteine;
- 1 tablet contains 600 mg of acetylcysteine;
- excipients: anhydrous citric acid, maltodextrin, sodium bicarbonate, orange flavoring, leucine, sodium saccharin.
Contraindication
Hypersensitivity to acetylcysteine or to any of the excipients.
Gastric and duodenal ulcer in the acute stage, hemoptysis, pulmonary hemorrhage.
Children under 12 years of age. However, the patient’s age under 12 years of age is not a contraindication to use in the treatment of paracetamol overdose.
Adverse reactions
The most common adverse reactions associated with oral administration of acetylcysteine are gastrointestinal reactions. Hypersensitivity reactions, including anaphylactic shock, anaphylactic/anaphylactoid reaction, bronchospasm, angioedema, rash and pruritus, have been reported less frequently.
Method of application
Adults and children aged 12 and over
Dissolve a 600 mg effervescent tablet in 1/3 cup of water and take once a day.
The duration of the course of treatment is determined individually by the doctor, depending on the nature of the disease (acute or chronic).
Paracetamol overdose
In the first 10 hours after taking the toxic substance, the drug “Rapira Efertab 600” should be taken as soon as possible at a rate of 140 mg/kg, then at a rate of 70 mg/kg every 4 hours for 1–3 days.
“Rapira Efertab 600” must be taken without delay, immediately after dissolution.
No interactions with food have been reported; there are no recommendations for use of the drug with respect to meals.
Application features
Use during pregnancy or breastfeeding
As a precautionary measure, the use of Rapira Efertab 600 should be avoided during pregnancy.
Before using the drug during pregnancy, the potential risks should be weighed against the expected benefits.
There is no information on the excretion of acetylcysteine and/or its metabolites into breast milk. A risk to the infant cannot be excluded.
A decision must be made whether to discontinue breastfeeding or to discontinue/abstain from the use of Rapira Efertab 600 taking into account the benefit of breastfeeding for the child and the benefit of therapy for the woman.
Children
Use in children over 12 years of age.
Ability to influence reaction speed when driving vehicles or other mechanisms
There is no evidence that acetylcysteine affects the ability to drive and use other mechanisms.
Overdose
There are no data on cases of overdose of medicinal forms of acetylcysteine intended for oral administration.
Volunteers took 11.2 g of acetylcysteine per day for three months without experiencing any serious side effects.
Acetylcysteine, when used at a dose of 500 mg/kg/day, does not cause overdose.
Overdose may manifest as gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
There is no specific antidote for acetylcysteine poisoning; therapy is symptomatic.
Interaction with other medicinal products and other types of interactions
Interaction studies were conducted only in adults.
The use of antitussives with acetylcysteine may increase sputum congestion due to suppression of the cough reflex.
Activated charcoal reduces the effectiveness of acetylcysteine.
Information on the inactivation of antibiotics by acetylcysteine has so far been obtained only in in vitro experiments with direct mixing of the substances. If it is necessary to use acetylcysteine and any oral drugs (including antibiotics) simultaneously, they should be taken at an interval of at least 2 hours. This does not apply to loracarbef.
Significant hypotension and temporal artery dilation have been reported with concomitant administration of nitroglycerin and acetylcysteine. If concomitant administration of nitroglycerin and acetylcysteine is necessary, patients should be monitored for hypotension, which may be severe. Patients should be warned of the possibility of headache.
Concomitant use of acetylcysteine and carbamazepine may result in a decrease in carbamazepine levels to subtherapeutic levels.
Impact on laboratory tests
Acetylcysteine may interfere with the colorimetric assay for salicylates and the determination of ketone bodies in urine.
Storage conditions
Keep the tube tightly closed. This medicinal product does not require any special temperature storage conditions. Keep out of the reach of children.
Be the first to review “Rapira Efertab 600 effervescent tablets 600 mg tube 10 pcs.” Cancel reply
You may also like








Reviews
There are no reviews yet.