🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more

$18.69 Original price was: $18.69.$16.99Current price is: $16.99.
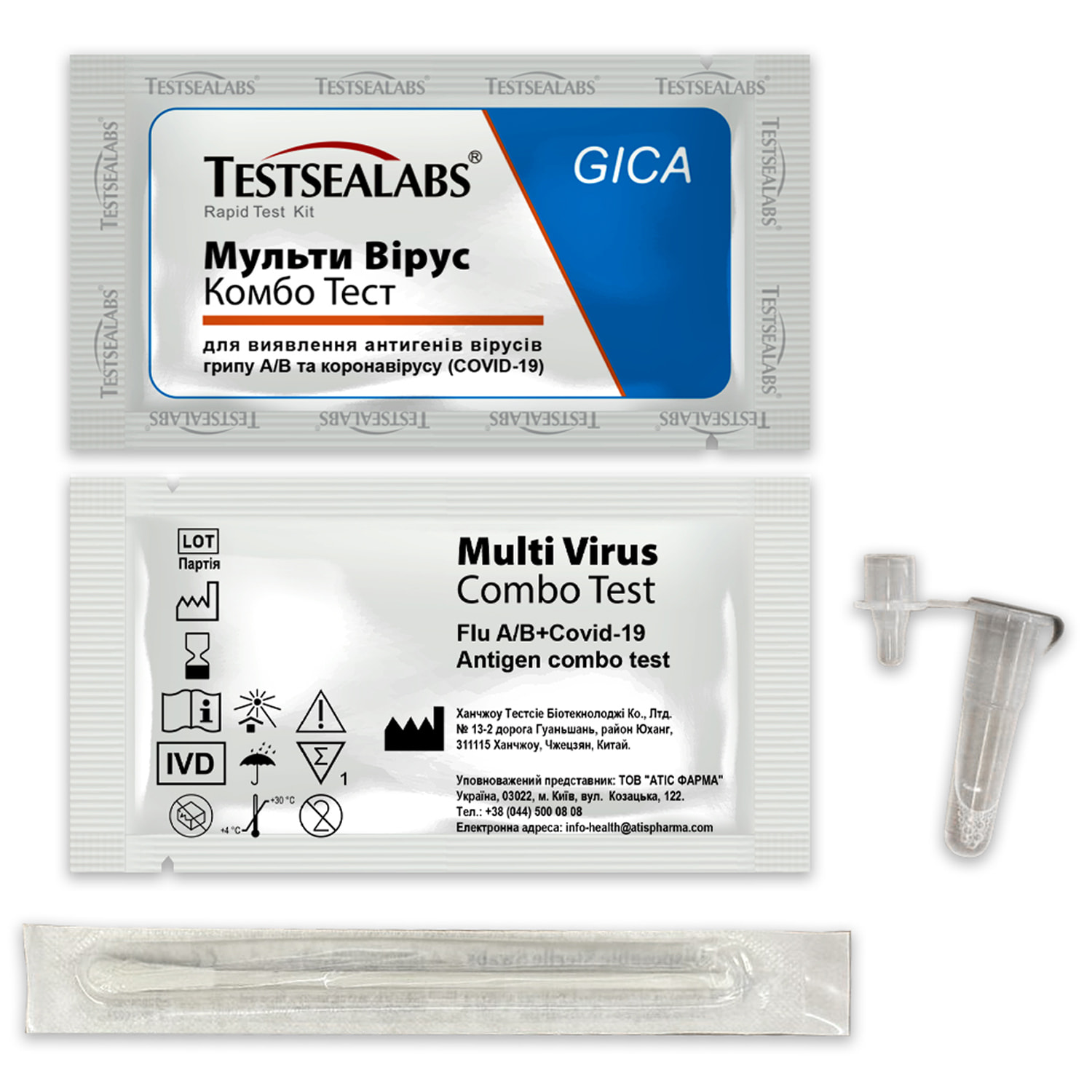
Testsealabs Multi Virus Combo Test for the detection of antigens of influenza A/B viruses and coronavirus (Covid-19) for self-testing nasal 1 pc.
$26.75
Multi Virus Combo rapid self-test detects Influenza A/B and COVID-19 antigens from nasal swab. Results in 10–15 minutes. For in vitro use.
-
— or —
Multi Virus Combo Test for the detection of antigens of influenza A/B viruses and coronavirus (COVID-19).
The Multi Virus Combo Test for the detection of influenza A/B and coronavirus (COVID-19) antigens is intended for self-testing and is a rapid immunochromatographic assay for the qualitative detection of influenza A/B and SARS-CoV-2 (COVID-19) antigens in nasal swab samples for the diagnosis of influenza and coronavirus viral infection.
For in vitro diagnostic use only. Intended for self-testing.
Multi Virus Combo Test for the detection of influenza A/B and coronavirus (COVID-19) antigens is a qualitative immunoassay based on a chromatographic immunological reaction on a membrane for the detection of influenza A/B and SARS-CoV-2 (COVID-19) antigens in a nasal swab sample.
The mechanism of action of the rapid test is based on the principle of sandwich immunochromatography with colloidal gold. The test strip consists of a sample collection pad, a reagent pad, a reaction membrane and an absorption pad. The reagent zone contains colloidal gold conjugated to detector monoclonal antibodies against SARS-CoV-2 or influenza A and B (depending on the type of test strip). The reaction membrane contains secondary antibodies to the corresponding viruses. The entire test strip is placed inside a plastic cassette.
After collecting the test sample and releasing it into the buffer solution, the conjugates contained in the reagent pad dissolve and migrate along each absorption pad with the sample due to the chromatographic effect. If SARS-CoV-2 antigen is present in the test sample, the complex formed between the anti-SARS-CoV-2 conjugate and the virus will be captured by the specific anti-SARS-CoV-2 monoclonal antibodies coated on the “T” zone of the test strip. If influenza A virus is present in the sample, the complex formed between the anti-Influenza A conjugate and the virus will be captured by the specific anti-Influenza A monoclonal antibodies coated on the “A” zone of the test strip. If the sample contains influenza B virus, the complex formed between the influenza B conjugate and the virus will be captured by the specific monoclonal antibodies against influenza B coated in the “B” zone of the test strip.
Features
The medical device contains an influenza A antibody, an influenza B antibody and an antibody to COVID-19-N as a capture reagent, another influenza A antibody, an influenza B antibody and an antibody to COVID-19-N as a detection reagent. Goat anti-mouse IgG is used in the control line system.
Advantages
The rapid test allows you to detect the following strains of three viruses:
- Influenza A: H1N1, H2N2, H3N2, H5N1, H5N3, H7N3, H7N8, H9N2, H10N4;
- Influenza B: all virus lines (Victoria, Yamagata);
- Coronavirus (SARS-CoV-2 or COVID-19): Alpha variant or “British” (B.1.1.7); Delta variant or “Indian” (B.1.617.2); Kappa variant (B.1.617.1) and “Omicron” (variants B.1.1.529, XBB.1.16, BA.2.12.1, BA.5.1.3).
At the user’s request, sample collection can be either nasal or from the middle nasal passage, which makes taking the material comfortable.
The rapid test includes an internal control of the procedure.
Result in 10-15 minutes (min).
Sensitivity, specificity, and overall convergence for influenza A virus antigen detection
- The overall agreement is 99.19%; 95% CI (95.13% – 99.99%);
- overall sensitivity is 100.0%; 95% CI (98.17% – 100.0%);
- overall specificity is 99.73%; 95% CI (98.34% – 99.99%).
Sensitivity, specificity, and overall convergence for influenza B virus antigen detection
- overall agreement is 99.07%; 95% CI (94.38% – 99.99%);
- overall sensitivity is 100.0%; 95% CI (98.17% – 100.0%);
- overall specificity is 99.72%; 95% CI (98.26% – 99.99%).
Sensitivity, specificity and overall convergence for Covid-19 virus antigen detection
- overall agreement is 99.56%; 95% CI (97.27% – 99.99%);
- overall sensitivity is 100.0%; 95% CI (99.35% – 100.0%);
- overall specificity is 99.85%; 95% CI (99.09% – 99.99%).
Indications for use
For the qualitative detection of influenza A/B virus antigens and SARS-CoV-2 coronavirus (COVID-19) in nasal swab samples for the diagnosis of influenza and coronavirus viral infection.
For in vitro diagnostic use only. Intended for self-testing.
Limitation
- The diagnostic result of this rapid test is not the only diagnostic criterion. If the test result does not match the clinical data, please contact your doctor for further consultation.
- Changes to the test procedure not specified in the instructions may reduce the accuracy of the result. The test is effective only when the instructions are followed.
- The rapid test is intended for the qualitative detection of influenza A/B antigens and SARS-CoV-2 coronavirus (COVID-19). The test is not intended for quantitative antigen determination or differentiation of virus strains.
- Test results should be interpreted in conjunction with clinical presentation and other laboratory tests. A positive result does not always correlate with a viral load sufficient to transmit infection. A negative result does not exclude infection with other pathogens. If in doubt, consult a doctor.
- Positive diagnostic results do not distinguish between influenza A and B viruses or the SARS-CoV-2 coronavirus (COVID-19).
- A false negative result may be obtained, especially if the diagnosis is not performed within the first 4 days after the onset of flu symptoms or within the first 7 days after the onset of SARS-CoV-2 coronavirus (COVID-19) symptoms.
- The effectiveness of the rapid test may be insufficient in the later stages of infection and in asymptomatic cases.
- A negative diagnostic result may occur if the antigen level in the sample is below the detection limit of the test.
- In case of a negative result due to symptoms, being in a high-risk environment, or professional necessity, it is recommended to repeat the test in 1-3 days.
- Due to the structural similarity of SARS-CoV-2 and SARS-CoV, cross-reactivity of the test with the SARS-CoV nucleoprotein is possible.
- A negative test result cannot be used to rule out other viral or bacterial infections unrelated to influenza A/B or COVID-19.
- If specific determination of the virus type or strain is required (e.g. for surveillance), additional testing should be performed in a specialized laboratory according to the recommendations of health authorities.
- Compliance with hygiene standards and anti-epidemic measures is mandatory, regardless of the test result.
Reservation
- For in vitro diagnostic use only. Read the instructions for use carefully before testing. Follow storage conditions.
- To obtain accurate results, diagnostics should be performed in accordance with the recommendations of this instruction.
- During diagnosis, persons under the age of 18 and persons with any disabilities must be supervised by adults or carry out the diagnosis only with their help.
- Wash your hands thoroughly before and after performing the diagnosis.
- If a third party collects the sample and performs the diagnosis, they should read the instructions for use and use personal protective equipment (disposable gloves, disposable mask) before starting the diagnostic procedure.
- All specimens should be handled as if they contain infectious agents. Dispose of them in accordance with local regulations for the disposal of biohazardous waste.
- Do not use this kit after the expiration date stated on the package.
- Use only undamaged rapid test. All components of this rapid test must remain sealed until used.
- Only the extraction buffer tube provided with this rapid test should be used. Do not substitute the extraction buffer solution for this rapid test with buffer solutions from other lots or components of other tests.
- Please note that excessive humidity and ambient temperature may negatively affect the analysis results.
- All components of the rapid test are disposable. Do not reuse them.
- The diagnostic procedure should occur immediately after opening and removing the rapid test from the individual packaging.
- Make sure that a sufficient amount of sample is used for diagnosis. Too much or too little sample may lead to incorrect results.
- Visually bloody or excessively viscous samples may lead to incorrect results.
- Never drink the buffer solution. If the buffer solution gets into your eyes or on your skin, rinse them thoroughly with water. If you feel unwell, seek medical attention immediately.
- The result should be read after 10 minutes (min). A result read after 20 minutes (min) is considered invalid.
- Do not disassemble the plastic case of the rapid test and do not touch the test strip.
Method of application
You should not eat, drink, or smoke (including e-cigarettes) for at least 30 minutes (min) before the test.
Diagnostics should be performed after the rapid test and the extraction tube with buffer solution have reached room temperature (+15 °C to +30 °C).
Before starting sample collection:
- Please read the instructions for use carefully.
- Prepare a clean work area for diagnostics.
- Make sure that all components of the rapid test kit are present and undamaged.
Sequence of material collection, sample preparation and diagnostics
ATTENTION! For delayed diagnosis, a sterile swab should be used in conjunction with a transport tube. Such a swab is not included with this medical device.
CAUTION! Do not let go of the swab during manipulations in the nostrils to prevent injury, inhalation, or entry of the swab into the sinuses.
Preparation for diagnostics
- The tampon is intended for single use. Do not use the tampon if the packaging has been damaged. The tampon should be used immediately after opening the packaging. Do not reuse the tampon.
- Unpack the swab. Carefully remove the swab from the packaging without touching the sample collection pad.
Sample collection
At the user’s request, sample collection can be either nasal or from the middle nasal passage.
Nasal sample.
- The tip of the swab should be inserted 2-4 centimeters (cm) into the nasal cavity (nostril) until resistance is felt. Do not insert the swab deeper if you feel strong resistance or pain.
- Slowly rotate the swab 5 times inside the nasal cavity. Repeat this process with the same swab for the other nasal cavity to ensure sufficient sample is collected.
Sample from the middle nasal passage (for better quality test sample).
- The tip of the swab should be inserted into the nasal cavity (nostril) with a light movement.
- Advance the swab parallel to the palate to a depth of 2-3 centimeters (cm) from the nasal opening. If the swab cannot be inserted to the appropriate depth (deviation of the nasal septum, other anatomical features, etc.), the attempt should be repeated through the other nostril.
- Having reached the measured distance, leave the swab for 2-3 seconds (s) to soak in nasal mucus, then remove the swab from the nasal cavity along its outer wall while making rotational movements.
Slowly rotate the swab out of the nasal cavity. Do not touch the soft tip to avoid contamination. The sample is now ready for preparation using the extraction buffer provided in the test kit.
If the swab is sufficiently saturated with secretions, it is not necessary to take a swab from the second nostril. In cases where the secretions are insignificant or difficult to obtain, it is permissible to take a swab from both nostrils with one swab.
CAUTION! Samples should be analyzed as soon as possible after collection for the best diagnostic efficiency. If immediate testing is not possible, to ensure the best results, avoid possible environmental contamination and sample deterioration, it is recommended to place the swab in a clean, previously unused plastic tube, maintaining the integrity of the sample, and store it at room temperature (+15 °C to +30 °C) for no more than 1 hour (h) for subsequent diagnosis. Make sure the tube is tightly closed. If the diagnosis is delayed by more than 1 hour (h), discard the sample; a new sample should be used for diagnosis.
If samples need to be transported, they should be packaged in accordance with local regulations for the transport of etiological agents.
Sample preparation and diagnostics
- Remove the protective foil from the buffer solution tube.
- Insert the swab with the sample vertically into the buffer tube. Rotate the swab in the buffer solution for 10 seconds (s), while pressing its head against the inner wall of the tube to improve antigen release.
- Remove the swab by squeezing the head inside the extraction tube. When removing the swab, squeeze out as much liquid as possible. Dispose of the used swab in accordance with local biohazard waste disposal regulations.
- Cap the tube; holding it vertically, place 3 (three) drops of the test sample into each sample well (S) of the test cassette. The sample wells are white and round in shape.
- Immediately after applying the sample to the test cassette, start the timer.
- Check the result after 10-15 minutes (min). If the result is not read after 20 minutes (min) or longer, it is considered invalid and retesting is recommended.
INTERPRETATION OF RESULTS
Negative
In the control zone for influenza A and B and coronavirus (COVID-19), a red band appears only opposite control “C”, other colored lines are absent. This means that no antigen of influenza A virus or influenza B virus or coronavirus (COVID-19) was detected in the sample.
The sample is likely not infected with influenza A/B and coronavirus (COVID-19) at the time of diagnosis. This does not guarantee complete absence of infection with influenza A/B or coronavirus (COVID-19). For individuals with a negative test result, laboratory PCR testing is recommended if symptoms are present. Please pay attention to symptoms and seek medical attention if symptoms persist.
Positive
Influenza A: A red line opposite the control “C” and in the test region “A” indicates a positive result for influenza A virus antigen.
Influenza B: A red line opposite the control “C” and in the test region “B” indicates a positive result for influenza B virus antigen.
Influenza A+B: A red band opposite the control “C” and in the test region “A” and “B” indicates a positive result for influenza A and influenza B virus antigen.
COVID-19: a red stripe opposite the control “C” and in the test zone “T” indicates a positive result for the SARS-CoV-2 virus antigen.
COVID-19 + Influenza A: A red band opposite the control “C”, in the test zone “A” and “T” indicates a positive result for influenza A virus antigen and SARS-CoV-2 virus antigen.
COVID-19 + Influenza B: A red band opposite the control “C”, in the test zone “B” and “T” indicates a positive result for influenza B virus antigen and SARS-CoV-2 virus antigen.
COVID-19+Influenza A+B: a red band opposite the control “C”, in the test zone “A”, “B” and “T” indicates a positive result for influenza A virus antigen, influenza B virus antigen and SARS-CoV-2 virus antigen.
One or more positive results indicate a high probability of infection with one or more viral agents and the possibility of infecting others. Please follow the instructions of the Ministry of Health, consult a doctor to determine the need for a confirmatory laboratory PCR test; seek medical attention if you feel unwell.
The intensity of the color in the test line region may vary depending on the concentration of viral antigens present in the specimen. Accordingly, any shade of color in the test line region should be considered positive.
Invalid
If the control line “C” does not appear in the result window after performing the test, the result is considered invalid.
It is recommended to re-read the instructions for use and repeat the diagnosis using a new rapid test. Do not use components from a previous rapid test. If the problem persists, immediately stop using the rapid test and contact an authorized representative (details are listed at the end of the instructions).
DO NOT MAKE ANY MEDICAL DECISIONS BASED ON THE DIAGNOSTIC RESULTS WITHOUT CONSULTING A DOCTOR!
WASTE DISPOSAL
After diagnosis, used rapid test components and personal protective equipment should be placed in a complete disposal bag and disposed of in accordance with local biohazard waste disposal regulations. Wash hands after completing all procedures.
Composition
Package contents:
- test cassette;
- extraction tube with buffer solution;
- tampon;
- instructions for use;
- package for recycling.
You may also like










Reviews
There are no reviews yet.