🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
🇦🇺 Australia
🇨🇦 Canada
🇨🇿 Czechia
🇩🇰 Denmark🇪🇪 Estonia
🇮🇪 Ireland
🇮🇱 Israel
🇮🇹 Italy
🇯🇵 Japan
🇲🇽 Mexico
🇵🇱 Poland
🇰🇷 South Korea
🇨🇭 Switzerland
🇬🇧 United Kingdom
🇺🇸 United States of Americaand more
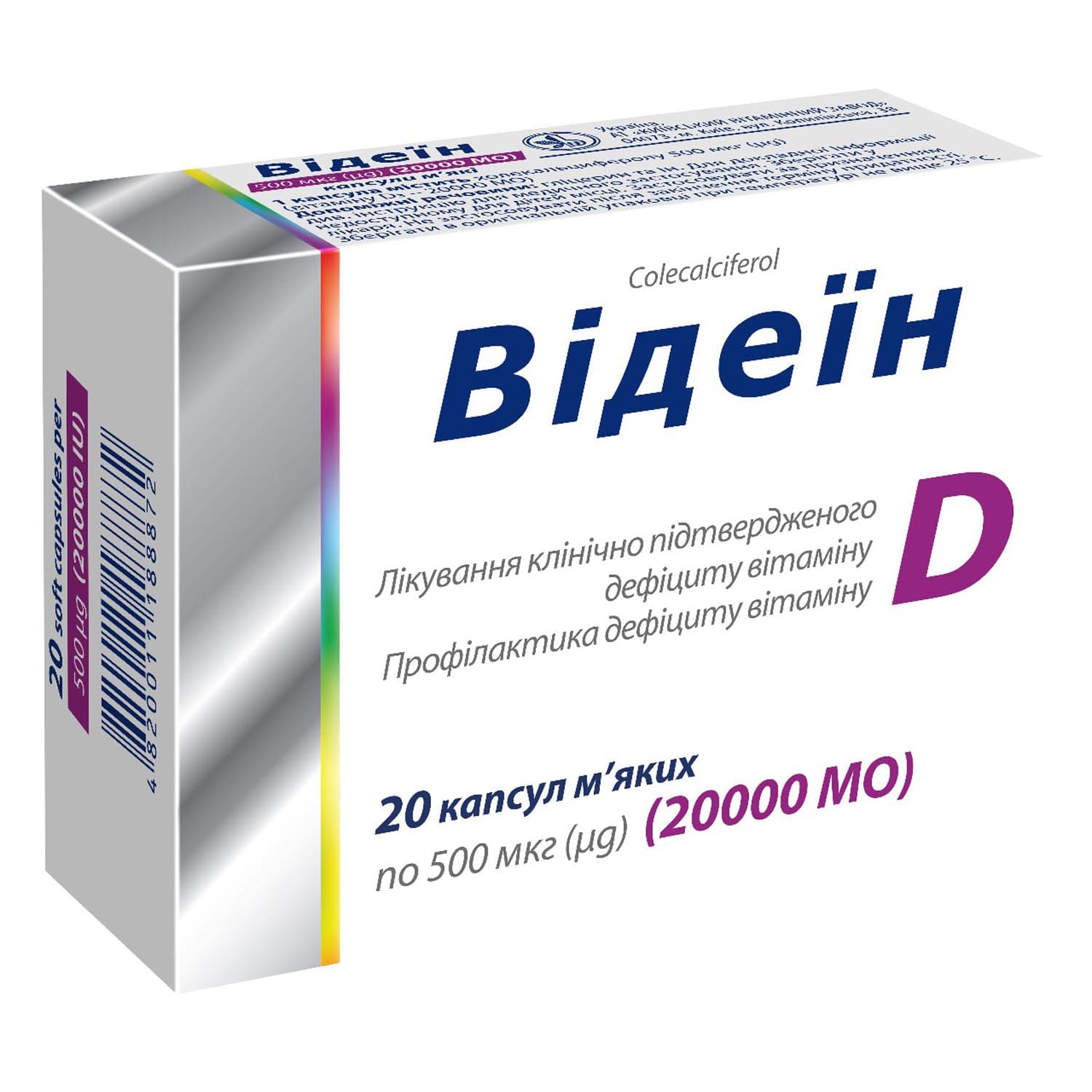
Videin soft capsules 5000 mcg (20,000 IU) 20 pcs.
$44.82
Videin vitamin D3 20,000 IU capsules treat and prevent vitamin D deficiency, support osteoporosis therapy, with clear dosing guidance and safety warnings.
-
— or —
Videin – vitamins. Preparations of vitamin D and its analogues.
Indications for use
- Use for the treatment of clinically confirmed vitamin D deficiency in adults.
- For the prevention of vitamin D deficiency in high-risk patients.
- As an adjunct to specific osteoporosis therapy in patients with vitamin D deficiency or at high risk of vitamin D deficiency.
Composition
- active ingredient: cholecalciferol;
- 1 capsule contains 500 mcg of cholecalciferol (vitamin D3 – 20,000 IU);
- excipients: α-tocopherol acetate, medium chain triglycerides;
- capsule shell: gelatin, glycerin.
Contraindication
- Hypersensitivity to the components of the drug.
- Hypercalcemia.
- Hypercalciuria.
- Hypervitaminosis D.
- Pseudohypoparathyroidism (vitamin D requirements may be lower than during periods of normal sensitivity to the vitamin, with the risk of prolonged overdose).
- Nephrolithiasis (urinary stone disease).
- Kidney failure.
- Sarcoidosis.
- Tuberculosis.
- Supplemental vitamin D (may lead to overdose).
Adverse reactions
Cardiovascular system – arrhythmia, arterial hypertension.
Digestive tract – constipation, flatulence, nausea, abdominal pain, diarrhea, loss of appetite, vomiting, dry mouth, dyspepsia.
Nervous system – headache, drowsiness, mental disorders, depression.
Urinary system – increased calcium levels in the blood and/or urine, urolithiasis and tissue calcification, uremia, polyuria.
Skin – hypersensitivity reactions, including urticaria, rash, itching
Musculoskeletal system – myalgia, arthralgia, muscle weakness.
Organs of vision – conjunctivitis, photosensitivity.
Method of application
For oral use.
Adults should take the capsule by swallowing it whole and drinking plenty of water, preferably during the main meal of the day.
The dosage of vitamin D depends on the severity of the disease and the patient’s response to treatment. Depending on the needs, possibilities and choice of the patient, daily, weekly or monthly dosing regimens can be offered. Lower dosage forms (e.g. 400 IU, 500 IU, 800 IU and 1000 IU) are suitable for daily vitamin D supplementation, while higher dosage forms, e.g. 20,000 IU capsules, contain an amount corresponding to weekly and monthly doses of vitamin D, which should be taken into account. The dosage is prescribed by the doctor in each individual case.
Dosage recommendations:
Initial treatment for vitamin D deficiency: 40,000 IU per week for 6–12 weeks.
Treatment of vitamin D deficiency and maintenance of its level: from 60,000 to 120,000 IU per month for a period of up to 6 months.
Prevention of vitamin D deficiency: 20,000 IU per week regardless of initial level from November to April.
Adjunct to specific osteoporosis therapy in patients with vitamin D deficiency or at risk of vitamin D deficiency: adults and the elderly: 20,000 to 40,000 IU per month in combination with a calcium supplement, if necessary.
Elderly people with a history of falls should avoid doses exceeding 24,000 IU per month.
Application features
Use during pregnancy or breastfeeding
During pregnancy, the drug Videin, 20,000 IU, can be prescribed only if there are clear indications, when it is absolutely necessary to eliminate vitamin D deficiency.
During pregnancy, vitamin D overdose should be avoided, as prolonged hypercalcemia can lead to a slowdown in the physical and mental development of the child and the appearance of supravalvular aortic stenosis and retinopathy in the child.
Vitamin D and its metabolites are excreted in breast milk. No cases of overdose in breastfed infants have been observed. However, this should be taken into account when prescribing additional vitamin D to the child. It is not recommended to prescribe vitamin D treatment in high doses, for example in capsules of 20,000 IU, to women who are breastfeeding.
Children
The use of the drug in children and adolescents (under 18 years of age) is not recommended due to the lack of data on the dosage regimen and the risk of choking when children take capsules. Instead, it is advisable to use drops or dissolvable tablets.
Ability to influence reaction speed when driving vehicles or other mechanisms
Videin, 20,000 IU, has no or negligible influence on the ability to drive or use machines. However, special caution is recommended when driving or operating machinery due to the possibility of developing adverse reactions from the nervous system.
Overdose
Vitamin D3 regulates calcium and phosphate metabolism – after overdose, hypercalcemia, hypercalciuria, renal calcifications and bone lesions occur, as well as changes in the cardiovascular system. Hypercalcemia occurs after the use of 50,000–100,000 IU of vitamin D3 per day.
Symptoms of overdose. Acute and chronic overdose of vitamin D3 can cause hypercalcemia, which can become persistent and life-threatening. Symptoms can be nonspecific and manifest as cardiac arrhythmia, thirst, dehydration, adynamia and impaired consciousness. In addition, chronic overdose can lead to calcium deposition in blood vessels and tissues.
In addition to increasing the phosphorus content in the blood serum and urine, overdose can also cause hypercalcemic syndrome, which subsequently leads to calcium deposition in the tissues and, in particular, in the kidneys (nephrolithiasis, nephrocalcinosis, renal failure), as well as in the blood vessels.
Symptoms of intoxication are not very characteristic and manifest as muscle weakness, loss of appetite, nausea, vomiting, initial frequent diarrhea, which is replaced by constipation, anorexia, shortness of breath, headache, myalgia, arthralgia, muscle weakness and constant drowsiness, arrhythmia, azotemia, polydipsia and polyuria, as well as (in the pre-terminal stage) dehydration, photosensitivity, pancreatitis, rhinorrhea, hyperthermia, decreased libido, conjunctivitis, hypercholesterolemia, increased transaminase activity, arterial hypertension, uremia. Common symptoms are pain in the muscles and joints.
Renal dysfunction develops with albuminuria, erythrocyturia and polyuria, increased potassium loss, hyposthenuria, nocturia and moderate increase in blood pressure.
In severe cases, corneal clouding is possible, less often – swelling of the optic nerve papilla, inflammation of the iris, up to the development of cataracts.
Kidney stones and calcifications in soft tissues such as blood vessels, heart, lungs, and skin may form.
Cholestatic jaundice rarely develops.
Characteristic biochemical abnormalities are hypercalcemia, hypercalciuria, and increased serum 25-hydroxycalciferol concentration.
Treatment. The appearance of symptoms of chronic vitamin D overdose may require forced diuresis, as well as the administration of glucocorticoids and calcitonin.
In the event of overdose, measures should be taken to eliminate the often chronic and potentially life-threatening hypercalcemia.
As a primary measure, vitamin D should be discontinued; normalization of calcium levels in hypercalcemia resulting from vitamin D intoxication takes several weeks.
Storage conditions
Store in the original packaging at a temperature not exceeding 25 °C.
Keep out of reach of children.
You may also like







Reviews
There are no reviews yet.