
Venorutinol gel 2% tube 40 g
$15.50
Venorutinol is a drug with P-vitamin activity and pronounced angioprotective properties. It reduces the permeability and fragility of capillaries, strengthens the walls of veins and capillaries, increases the tone of smooth muscles of venous blood vessels. It has anti-edematous, anti-inflammatory and analgesic effects.
Composition and form of release
Composition
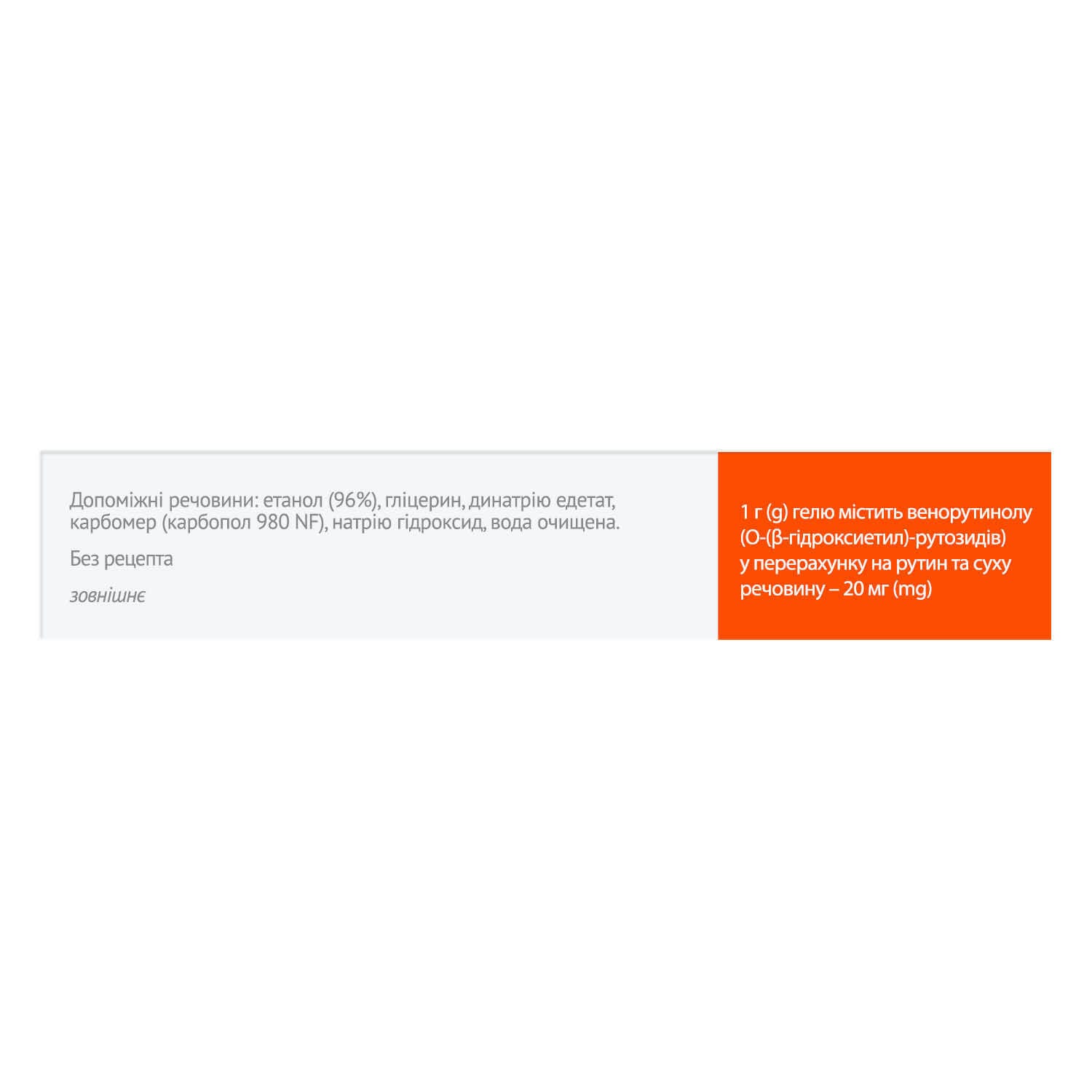
- active ingredient: 1 g of gel contains 20 mg of venorutinol (O-(β-hydroxyethyl)-rutosides), calculated as rutin and dry matter;
- excipients: ethanol (96%), glycerol, disodium edetate, carbomer (carbopol 980 NF), sodium hydroxide, purified water.
Release form
Gel.
Pharmacological properties
Troxerutin is a drug with p-vitamin activity and a pronounced angioprotective effect. The drug reduces vascular tissue permeability and capillary fragility, promotes normalization and improvement of tissue trophism, reduces stagnant phenomena in veins and paravenous areas, exhibits anti-edematous and anti-inflammatory effects, increases the tone of smooth muscles of venous blood vessels; participates in redox processes; stabilizes the activity of hyaluronic acid in cell membranes, blocking the activity of hyaluronidase; increases the density of the vascular wall, reduces exudation of the liquid part of the plasma and diapedesis of formed blood elements. By reducing the manifestations of exudative inflammation in the vessels, troxerutin limits the adhesion of platelets to the surface of the vascular wall. After taking the capsule inside, the drug is well absorbed in the gastrointestinal tract. Cmax of the active substance in plasma is noted on average 2 hours after ingestion. It penetrates the blood-brain barrier well. It is metabolized in the liver to form several metabolites. It is partially excreted unchanged in the urine and bile.
Indication
- prevention and treatment of varicose veins;
- pain and swelling of the extremities caused by venous insufficiency;
- trophic disorders and ulcers in chronic venous insufficiency;
- thrombosis, thrombophlebitis;
- post-phlebitic conditions;
- closed injuries (including sports injuries).
Application
The drug is used externally. The gel is applied in a thin layer in the morning and evening to the affected areas with light massage movements until it is completely absorbed into the skin. The course of treatment depends on the severity of the disease and can last up to 20 days.
Contraindication
- hypersensitivity to the components of the drug;
- age up to 14 years.
Side effects
In some cases, local hypersensitivity reactions occur – urticaria, dermatitis, eczema. Most of these manifestations quickly disappear after discontinuation of the drug.
Special instructions
Do not apply to damaged skin areas, avoid contact with eyes and mucous membranes. Can be applied under an occlusive dressing.
Use during pregnancy and breastfeeding.
Should not be used in the first 3 months of pregnancy.
In the II and III trimesters of pregnancy and during breastfeeding, the drug may be used under the supervision of a physician if the expected benefit to the mother outweighs the potential risk to the fetus/child.
If the drug is used in breastfeeding women, breastfeeding is recommended to be discontinued.
Children: The efficacy and safety of the drug in children have not been studied.
Ability to influence the reaction rate when driving vehicles or working with other mechanisms. There is no data on the ability of the drug to influence the reaction rate when driving vehicles or working with other mechanisms.
Interactions
The drug enhances the effect of ascorbic acid in strengthening the structure and reducing the permeability of the vascular wall.
Overdose
Cases of overdose with Venorutinol are unknown.
Treatment. In case of accidental ingestion of a large amount of gel, induce vomiting and consult a doctor. If indicated, peritoneal dialysis should be used. Treatment is symptomatic.
Storage conditions
In a dry place protected from light at a temperature of 15-25 °C.








Reviews
There are no reviews yet.